Unlocking the Potential of Rechargeable Lithium Batteries
January 10, 2014
What Is The Scientific Achievement?
Nearly all high energy density cathodes for rechargeable lithium batteries are well-ordered materials, in which lithium and other cations occupy distinct sites. Disordered materials are generally disregarded as cathodes because lithium diffusion tends to be limited in those structures. The outstanding performance of a newly developed electron material (Li1.211Mo0.467Cr0.3O2) contradicts this assumption. In this work, we show that lithium diffusion can be facile in this disordered material. Using ab-initio computations, we demonstrate that this unexpected behavior is due to percolation of certain diffusion channels that are active in disordered structures, but which are unique to Li-excessive materials. This leads to a unified understanding of the high performance in both layered and Li-excessive materials, and opens up an exciting new direction for designing disordered electrode materials with high capacity and high energy density.
Why Does This Matter?
Over the years, researchers have believed that high energy density cathodes for rechargeable lithium batteries should be well-ordered materials, in which lithium and other cations occupy distinct sites. We found an unexpectedly high performance associated with a newly developed electrode material, Li1.211Mo0.467Cr0.3O2 (LMCO). We demonstrated that the origin of this high performance is from Li diffusion along channels in the structure, which allows the Li ions to move in and out of the electrode quickly.
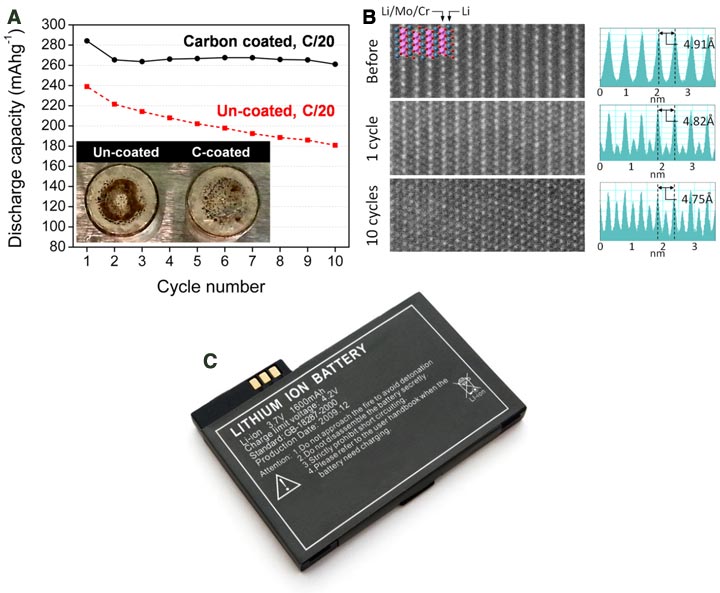
(A) The discharge capacity retention of LMCO/C (black) and LMCO (red) during cycling at room temperature. The two inset images show Li metal anode foils from LMCO and LMCO/C half cells after ten cycles. (B) The STEM images along the [010] zone axis in a LMCO/C particle before and after 1 and 10 cycles(left). The corresponding line profiles of the Z-contrast information with the measured spacing between (Li/Mo/Cr) layers (right). (c) Our research opens up exciting new opportunities for the development of next generation batteries.
What Are The Specifics?
- CFN Capabilities: Atomic resolution images of the material’s structure were obtained using the CFN’s advanced electron microscopes. These images demonstrated an increase in disorder with increasing numbers of charge/discharge cycles.
- The conventional wisdom is that high energy density cathodes for rechargeable lithium batteries should be well-ordered materials, with lithium and other ions moving through distinct sites in the structure. The observation that a disordered material, such as LMCO, can exhibit outstanding performance provides new insight into the mechanism of lithium diffusion in materials, and opens up an exciting new direction for battery electrode design.
Reference
Unlocking the potential of disordered oxides for rechargeable lithium batteries
Jinhyuk Lee1, Alexander Urban1, Xin Li1, Dong Su2, Geoffroy Hautier1, and Gerbrand Ceder1*
1 Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA.
2 Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York, 11973, USA.
Acknowledgment of Support
This work was supported by the Robert Bosch Corporation and Umicore Specialty Oxides and Chemicals, as well as by Samsung Scholarship. Computational resources from the National Energy Research Scientific Computing Center (NERSC) and from the Extreme Science and Engineering Discovery Environment (XSEDE) are gratefully acknowledged. The STEM work carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.
2014-4664 | INT/EXT | Newsroom