New Catalyst for Making Methanol from Methane
November 15, 2016
By Ping Liu, Sanjaya Senanayake, and José A. Rodriguez
Our group has developed a catalyst for converting methane, the main component of abundant natural gas, directly to methanol at fairly low temperatures. Refining this catalyst could lead to a major commercial breakthrough—an inexpensive way to convert methane into methanol and other fuels or feedstocks for the synthesis of commodity goods such as plastics, paints, and textiles.
Currently converting methane to methanol is a two-step process, with the first step being extremely energy-intensive, and therefore expensive. Many scientists have been exploring direct, single-step methods. So far, these methods have been inefficient, resulting in low methanol production because the reaction tends to proceed all the way to carbon monoxide or carbon dioxide—effectively “eating up” the hydrocarbon intermediates that could serve as building blocks for useful products. These reactions also run at high temperatures, around 600K (620°F) and the cost is high. Lowering the temperature appears to be a key to controlling the reaction.
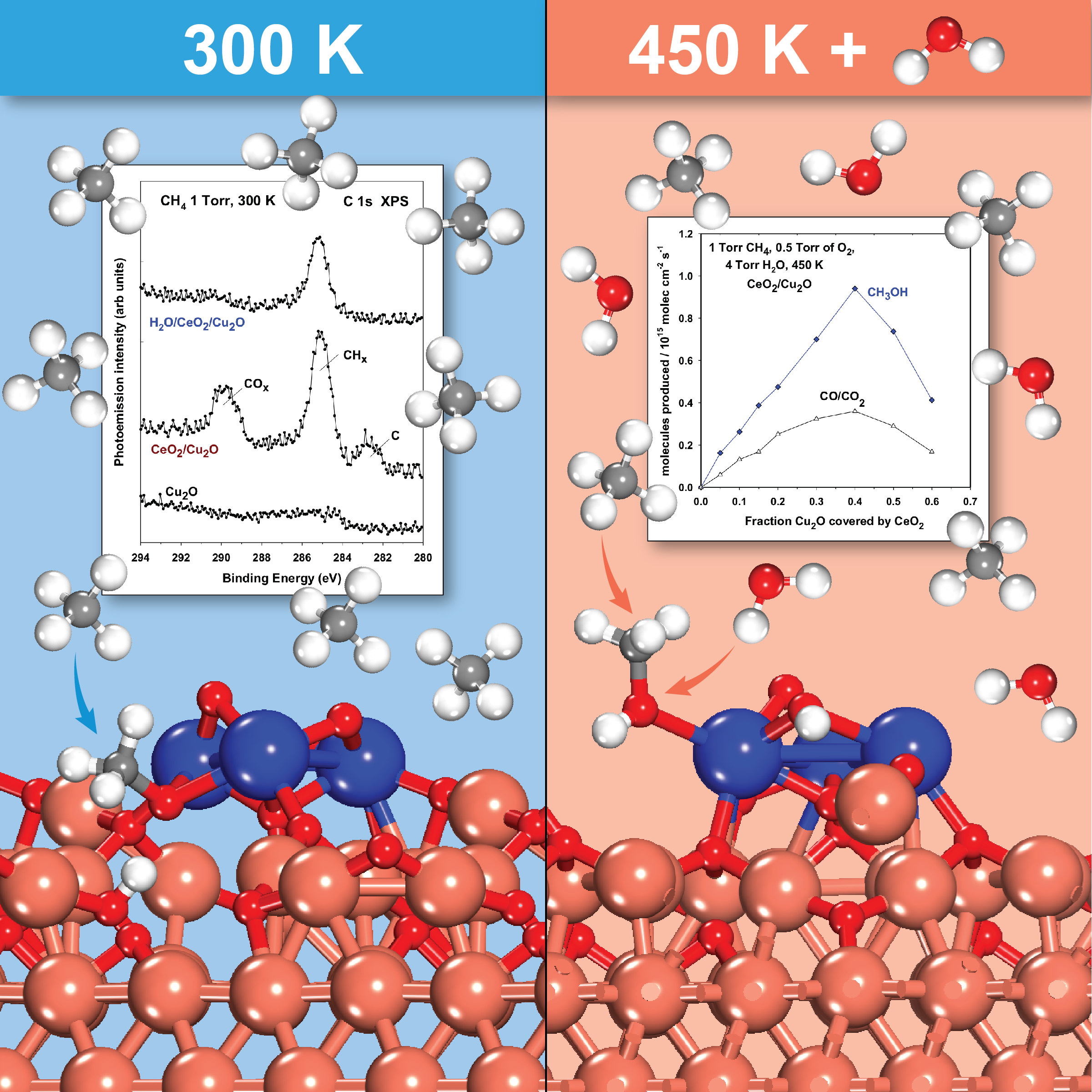
Our approach uses a catalyst made of inexpensive cerium dioxide (ceria) particles on a copper-oxide surface. At room temperature, hydrogen from the methane binds to oxygen in the ceria particles, freeing reactive hydrocarbon intermediates. Adding water provides hydroxide (OH) groups that react with these hydrocarbon intermediates at a temperature of just 350°F to produce methanol at high yield. The hydroxide groups also block side reactions that can break down the hydrocarbon intermediates.
We used computational resources at the Center for Functional Nanomaterials (CFN), a DOE Office of Science User Facility, to model these interactions. These simulations helped us identify the underlying mechanism and the active sites that enable such high activity at low temperature and the selective production of methanol.
 enlarge
enlarge
Reaction schematic: At room temperature (300 Kelvin, or 80° Fahrenheit), hydrogen from the methane binds to oxygen in the ceria particles, freeing reactive hydrocarbon intermediates. Adding water provides hydroxide (OH) groups that react with the hydrocarbon intermediates at a temperature of just 450K (350°F) to produce methanol at high yield.
Based on this fundamental understanding, we are exploring other low-cost metal compounds to see if we can improve this catalyst in terms of methane conversion and methanol selectivity.
This research, published in JACS Communications, helps identify key principles for low-temperature catalysts that could eventually be used to capture methane and convert it to methanol at natural gas wells, thus producing an easy-to-transport, stable liquid. But more work will be needed to turn the current model catalysts into practical powder catalysts that are inexpensive, durable, selective, and active enough for commercial applications.
This work was funded by the DOE Office of Science (BES).
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
2016-6694 | INT/EXT | Newsroom