- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
High Activity of Ce1-xNixO2-y for H-2 Production through Ethanol Steam Reforming: Tuning Catalytic Performance through Metal-Oxide Interactions
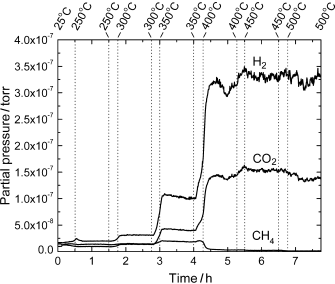 The
importance of the oxide: Ce0.8Ni0.2O2−y
is an excellent catalyst for ethanol steam reforming Metal–oxide
interactions perturb the electronic properties of the small particles of
metallic nickel present in the catalyst under the reaction conditions and
thus suppress any methanation activity. The nickel embedded in ceria induces
the formation of O vacancies, which facilitate cleavage of the
O
The
importance of the oxide: Ce0.8Ni0.2O2−y
is an excellent catalyst for ethanol steam reforming Metal–oxide
interactions perturb the electronic properties of the small particles of
metallic nickel present in the catalyst under the reaction conditions and
thus suppress any methanation activity. The nickel embedded in ceria induces
the formation of O vacancies, which facilitate cleavage of the
O![]() H
bonds in ethanol and water. Ce0.8Ni0.2O2−y
is an excellent catalyst for ethanol steam reforming. It is less expensive
than Rh/CeO2 and has a higher catalytic activity. Under the
reaction conditions, Ce0.8Ni0.2O2−y
contains small particles of nickel dispersed on partially reduced
nickel-doped ceria. Metal–oxide interactions perturb the electronic
properties of Ni and suppress its activity for methanation; at the same
time, the nickel embedded in ceria induces the formation of O vacancies that
facilitate the cleavage of the
O
H
bonds in ethanol and water. Ce0.8Ni0.2O2−y
is an excellent catalyst for ethanol steam reforming. It is less expensive
than Rh/CeO2 and has a higher catalytic activity. Under the
reaction conditions, Ce0.8Ni0.2O2−y
contains small particles of nickel dispersed on partially reduced
nickel-doped ceria. Metal–oxide interactions perturb the electronic
properties of Ni and suppress its activity for methanation; at the same
time, the nickel embedded in ceria induces the formation of O vacancies that
facilitate the cleavage of the
O![]() H
bonds in ethanol and water. These studies show the importance of both the
metal and the oxide phase in catalysts for ethanol steam reforming. Both
phases must be taken into consideration when trying to improve catalyst
performance.
H
bonds in ethanol and water. These studies show the importance of both the
metal and the oxide phase in catalysts for ethanol steam reforming. Both
phases must be taken into consideration when trying to improve catalyst
performance.
Ref: Zhou, G., Barrio, L., Agnoli, S., Senanayake, S.D., Evans, J., Kubacka, A., Estrella, M., Hanson, J.C., Martinez-Arias, A., Fernandez-Garcia, M., and Rodriguez, J.A. Angewandte Chemie-International Edition, 2010. 49(50): p. 9680-9684. DOI:10.1002/anie.201004966




