- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
Theoretical catalyst optimization of WGS catalysts: Cu(111) supported oxide nanostructures
Theoretical catalyst
optimization of WGS catalysts: Cu(111) supported oxide nanostructures
The key for theoretical catalyst optimization is to identify the key factors, which are able to scale well with the overall activity. What we learn from our studies of working catalysts set up a strong basis for theoretical optimization of better WGS catalysts. What we learn from our mechanistic studies is that a good WGS catalyst should be active enough to dissociate water, but still being able to oxidize and remove CO efficiently. Pure Cu and Au systems cannot accomplish the first task well. One effective way to optimize the WGS activity of Au and Cu is to use an oxide, making a bifunctional catalyst: CO interacts with the metals, the oxides help the water dissociation, and the reaction occurs at the metal-oxide interface. Indeed, our sensitivity analysis based kinetic modeling showed that the calculated barrier for water dissociation is the key parameter for Cu-Au-based systems we studied. The lower the barrier for water dissociation is, the higher the WGS activity will be (Figure on the left).
One of our interested system is Oxide/Cu(111). Two different models are considered: one is oxide chain (MxOx), which is constructed to represent the brim of relatively big oxide islands on metal surfaces, which was observed in STM. The other is oxide trimmer (M3O3x) to simulate the relatively small particles of oxides. Our first-cycle optimization is based on the key parameter, reaction energy for H2O dissociation. Several systems [e.g. Mg3O3, MoO3, Zr3O6/Cu(111)] were predicted to much more active than Cu, Yet, the second-cycle optimization based on the CO reaction has to be performed to confirm the predictions, as these data point are not in range of the previously verified by experiments, solid line, but in the extrapolated dash line region (Figure on the right). A good WGS catalyst should be active enough to dissociate H2O, but still being able to oxidize and remove CO efficiently. Our established correlation provides a strong basis to theoretical optimization of metal-oxide catalysts for the WGS reaction.
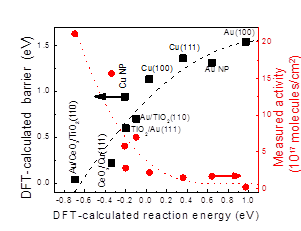
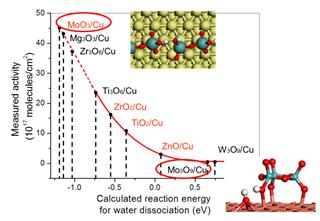
Ref: P. Liu, The Journal of Physical Chemistry C, 116 (2012) 25337-25343; A.B. Vidal, P. Liu, Physical Chemistry Chemical Physics, 14 (2012) 16626-16632.




