- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Catalysis: Reactivity and Structure
Unique Properties of Ceria Nanoparticles Supported on Metals: Novel Inverse Ceria/Copper Catalysts for CO Oxidation and the Water-Gas Shift Reaction
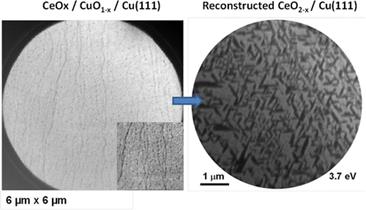 This
manuscript describes the properties of inverse catalysts composed of
CeOx nanoparticles supported on Cu(111) or CuOx/Cu(111)
as determined through the methods described above. Ceria is an important
material for redox chemistry because of its interchangeable oxidation states
(Ce4+ and Ce3+). Cu(111), meanwhile, is a standard
catalyst for reactions such as CO oxidation and the water-gas shift (WGS).
This metal serves as an ideal replacement for other noble metals that are
neither abundant nor cost effective. To prepare the inverse system we
deposited nanoparticles (2–20 nm) of cerium oxide onto the Cu(111) surface.
During this process, the Cu(111) surface grows an oxide layer that is
characteristic of Cu2O (Cu1+). This oxide can
influence the growth of ceria nanoparticles. Evidence suggests
triangular-shaped CeO2(111) grows on Cu2O(111)
surfaces while rectangular CeO2(100) grows on Cu4O3(111)
surfaces. We used the CeOx/Cu2O/Cu(111) inverse system
to study two catalytic processes: the WGS (CO + H2O → CO2
+ H2) and CO oxidation (2CO + O2 → 2CO2).
We discovered that the addition of small amounts of ceria nanoparticles can
activate the Cu(111) surface and achieve remarkable enhancement of catalytic
activity in the investigated reactions. In the case of the WGS, the CeOx
nanoparticle facilitated this process by acting at the interface with Cu to
dissociate water. In the CO oxidation case, an enhancement in the
dissociation of O2 by the nanoparticles was a key factor. The
strong interaction between CeOx nanoparticles and Cu(111) when
preoxidized and reduced in CO resulted in a massive surface reconstruction
of the copper substrate with the introduction of microterraces that covered
25–35% of the surface. This constitutes a new mechanism for surface
reconstruction not observed before. These microterraces helped to facilitate
a further enhancement of activity towards the WGS by opening an additional
channel for the dissociation of water. In summary, inverse catalysts of CeOx/Cu(111)
and CeO2/Cu2O/Cu(111) demonstrate the versatility of a
model system to obtain insightful knowledge of catalytic processes. These
systems will continue to offer a unique opportunity to probe key catalytic
components and elucidate the relationship between structure and reactivity
of novel materials and reactions in the future.
This
manuscript describes the properties of inverse catalysts composed of
CeOx nanoparticles supported on Cu(111) or CuOx/Cu(111)
as determined through the methods described above. Ceria is an important
material for redox chemistry because of its interchangeable oxidation states
(Ce4+ and Ce3+). Cu(111), meanwhile, is a standard
catalyst for reactions such as CO oxidation and the water-gas shift (WGS).
This metal serves as an ideal replacement for other noble metals that are
neither abundant nor cost effective. To prepare the inverse system we
deposited nanoparticles (2–20 nm) of cerium oxide onto the Cu(111) surface.
During this process, the Cu(111) surface grows an oxide layer that is
characteristic of Cu2O (Cu1+). This oxide can
influence the growth of ceria nanoparticles. Evidence suggests
triangular-shaped CeO2(111) grows on Cu2O(111)
surfaces while rectangular CeO2(100) grows on Cu4O3(111)
surfaces. We used the CeOx/Cu2O/Cu(111) inverse system
to study two catalytic processes: the WGS (CO + H2O → CO2
+ H2) and CO oxidation (2CO + O2 → 2CO2).
We discovered that the addition of small amounts of ceria nanoparticles can
activate the Cu(111) surface and achieve remarkable enhancement of catalytic
activity in the investigated reactions. In the case of the WGS, the CeOx
nanoparticle facilitated this process by acting at the interface with Cu to
dissociate water. In the CO oxidation case, an enhancement in the
dissociation of O2 by the nanoparticles was a key factor. The
strong interaction between CeOx nanoparticles and Cu(111) when
preoxidized and reduced in CO resulted in a massive surface reconstruction
of the copper substrate with the introduction of microterraces that covered
25–35% of the surface. This constitutes a new mechanism for surface
reconstruction not observed before. These microterraces helped to facilitate
a further enhancement of activity towards the WGS by opening an additional
channel for the dissociation of water. In summary, inverse catalysts of CeOx/Cu(111)
and CeO2/Cu2O/Cu(111) demonstrate the versatility of a
model system to obtain insightful knowledge of catalytic processes. These
systems will continue to offer a unique opportunity to probe key catalytic
components and elucidate the relationship between structure and reactivity
of novel materials and reactions in the future.
Senanayake, S.D., Stacchiola, D., and Rodriguez, J.A. Accounts of Chemical Research, 2013. 46(8): p. 1702-1711. DOI: 10.1021/ar300231p




