- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Increasing PtML activity by reducing O-BE
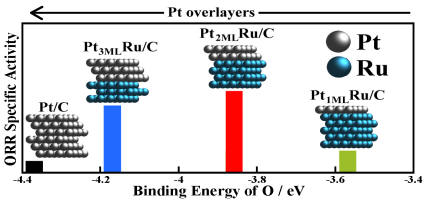
ORR activity vs. binding energy of O on Pt as a function of layerthickness
Tuning of the catalytic activity of Ru@Pt core-shell nanoparticles for the ORR can be controlled by varying the shell thickness. Pt monolayers were deposited on carbon-supported Ru nanoparticles on rotating disk electrode technique. The Pt mass and specific ORR activities greatly depend on the number of Pt monolayers, and the optimum activity occurs with two Pt monolayers. Density functional theory calculations showed that Pt overlayers destabilize O* and OH* with respect to pure Pt, leading to more favorable hydrogenation kinetics. However, with only a single Pt overlayer, the destabilization is too strong, and O−O bond breaking becomes rate limiting. Two to three Pt monolayers supported on the Ru cores nanoparticles lead to increased activity. This work demonstrates that one can modulate the ORR activity of Pt monolayers supported on other metals by eliminating a part of the ligand effect by increasing the thickness of the Pt shell on top of the supporting metal surface.
Journal of Physical Chemistry C, 2013, 117, 1748-1753.




