- Home
-
Research Groups
Division Groups
- Artificial Photosynthesis
- Catalysis: Reactivity & Structure
- Electrochemical Energy Storage
- Electron- and Photo-Induced Processes for Molecular Energy Conversion
- Neutrino and Nuclear Chemistry
- Surface Electrochemistry and Electrocatalysis
Associated Groups
- Catalysis for Alternative Fuels Production
- Nanostructured Interfaces for Catalysis
- Structure and Dynamics of Applied Nanomaterials
- People
- Operations
- News
- Events

Surface Electrochemistry and Electrocatalysis
Tensile strain increases the activity of PtML for oxidation of ethanol and methanol
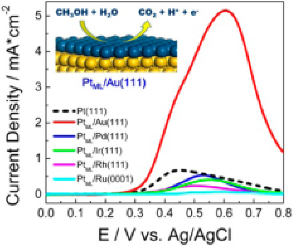
Effect of support-induced strain on the rate of methanol oxidation on PtML
Slow oxidation of methanol and ethanol are still major obstacle to commercialization of direct methanol and ethanol fuel cells. We demonstrated an important possibility for enhancing catalyst activity for oxidation of organic molecules. A model catalyst study shows that expansive strain caused by an underlying Au substrate can dramatically improve the activity of PtML for methanol, and ethanol oxidation. This is supported by DFT calculations. Co-catalysts such as Ru, Ir, SnO2, RhSnO2 further improve the performance of PtML due to the bi-functional mechanism. In situ IR spectroscopies show that RhSnO2/PtML/Au catalyst has a high selectivity in oxidizing ethanol to CO2. This electrocatalyst, consisting of one Pt monolayer (one atom thick layer) placed on extended or nanoparticle surfaces having the activity and selectivity for the oxidation of alcohol molecules that can be controlled with platinum-support interaction. The suitably expanded Pt monolayer (i.e., Pt/Au(111)) exhibits a factor of 7 activity increase in catalyzing methanol electrooxidation relative to Pt(111). Sizable enhancement is also observed for ethanol electrooxidation. Furthermore, a correlation between substrate-induced lateral strain in a Pt monolayer and its activity/selectivity is established and rationalized by experimental and theoretical studies. The knowledge we gained with single-crystal model catalysts was successfully applied in designing real nanocatalysts. These findings for alcohols are likely to be applicable for the oxidation of other classes of organic molecules.
Catalysis Today, 202 (2013) 50-54.




