
Cleaner Fuel Sources
Brookhaven scientists are exploring a range of clean-energy strategies with the aim of moving toward a net-zero carbon future. Examples include rewiring plants’ biochemical pathways to produce biobased fuels; developing catalysts that mimic plants’ ability to capture sunlight and transform carbon dioxide and water into fuel; and developing catalysts that transform carbon dioxide, waste methane, and other raw materials into useful products.
Designing catalysts to make alternative, cleaner fuels and other chemicals
Brookhaven chemists are exploring a wide range of catalysts that could enable the generation of useful chemicals and fuels from carbon dioxide and other greenhouse gases. These catalysts are like molecular deal makers; they bring chemical reactants together and make it easier for reactions to take place. Scientists use tools like Brookhaven’s National Synchrotron Light Source II (NSLS-II) and Center for Functional Nanomaterials (CFN) to understand what’s happening at the atomic level so they can use that information to design catalysts that are more efficient, less expensive, operate under more favorable conditions—or all three. Some reactions currently being explored:
- Turning atmospheric CO2 into useful chemicals and fuels by reacting it with “green” hydrogen generated using a renewable electricity source. This process could be carbon-neutral, where the CO2 used becomes CO2 again, but levels don’t increase.
- Using renewable energy to split water (electrolysis) to make hydrogen fuel, then use the fuel in fuel cell vehicles, making water again. This process has no carbon emissions. Brookhaven scientists are also designing and optimizing electrode materials for solar water splitting.
- Identifying the chemical structure of pollutant-causing sulfur and nitrogen atoms in hydrocarbon compounds found in petroleum. Nitrogen and sulfur oxides are two major pollutants generated from the combustion of fossil fuels.
- Using methane from landfills or vented at oil wells to generate hydrogen and syngas, a raw material for making many industrially important products we now derive from petroleum. While the carbon emitted by using the chemicals would eventually be returned to the atmosphere, it would have provided a value that would have been lost if just emitted or burned without being used.
Capabilities
Cleaner Fuel News

Finding the Catalyst for a More Sustainable Future
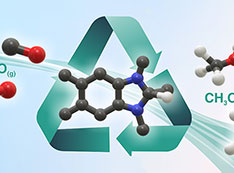
Recyclable Reagent and Sunlight Convert Carbon Monoxide into Methanol

When Plants Flower: Scientists ID Genes, Mechanism in Sorghum

Toshifumi Sugama Honored for Contributions to Geothermal Industry

Department of Energy Announces $112.4 Million for Research to Support National Biopreparedness and Response

Brookhaven Lab chemist Javier Concepcion and Lei Wang, a graduate student at Stony Brook University, devised a scheme for assembling light-absorbing molecules and water-splitting catalysts on a nanoparticle-coated electrode. The result: production of hydrogen gas fuel via artificial photosynthesis and a platform for testing different combos to further improve efficiency.
Catalysts for artificial photosynthesis, the ultimate clean energy source
Plants on earth harvest far more energy than is consumed by all human societies. Using energy from sunlight, they transform pure water and carbon dioxide into the sugars they use as fuel. Brookhaven chemists are working to mimic all or part of this process by developing catalysts that use sunlight to generate storable and transportable chemical fuels, ideally carbon-based fuels derived from CO2 in the atmosphere. In addition to transforming intermittent sunlight into an always-available energy source, this process would create a closed cycle of CO2 capture, conversion, storage, transport, and use, with the CO2 being emitted again when the fuel is burned. Such a closed cycle would recycle CO2 from the atmosphere rather than raising levels further (as burning fossil fuels does).

Brookhaven biochemist John Shanklin (center) with Hui Liu (left) and postdoc Zhiyang Zhai with tobacco plants used in a study to decipher a link between plants' sugar balance and oil production processes.
Rewiring plants to make fuels and bioproducts
Through photosynthesis, plants use sunlight and the atmospheric greenhouse gas CO2 to fuel their growth and development. Brookhaven biologists are working to understand the functions and regulation of plant genes involved in these biochemical pathways, particularly those that drive plants’ production of useful products like oils. Armed with this basic scientific knowledge, their goal is to “rewire” plants to produce abundant, sustainable quantities of key resources we now derive from petroleum-based chemicals—including oils that can be used as biofuels and raw materials for making products such as paints, lubricants, and plastics. Genomic and genetic studies will identify traits that enable such bioenergy/bio-product crops (e.g., “oilcane,” modified sugarcane that accumulates oil) to grow on marginal land, so they don’t compete for the fertile soil needed to grow the nation’s food supply.
Capabilities
- Expertise in genomics, biochemical genetics, computational modeling, and metabolic engineering at Brookhaven and at DOE’s Joint Genome Institute and Environmental Molecular Sciences Laboratory
- Access to world-leading structure and imaging capabilities at the National Synchrotron Light Source II and Center for Functional Nanomaterials




