
Net-Zero Carbon
Brookhaven scientists are exploring a range of clean-energy strategies with the aim of moving toward a net-zero carbon future. Examples include rewiring plants’ biochemical pathways to produce biobased fuels; developing catalysts that mimic plants’ ability to capture sunlight and transform carbon dioxide and water into fuel; and developing catalysts that transform carbon dioxide, waste methane, and other raw materials into useful products.
Catalysis for carbon conversion
Chemists at Brookhaven Lab are investigating processes that will help reduce the levels of carbon-based greenhouse gases and their harmful effects on Earth’s climate.
We are leveraging our expertise in both fundamental and applied science to advance carbon conversion chemistry that will reduce the emission of carbon dioxide (CO2), methane, or other greenhouse gases into the atmosphere by converting them into value-added fuels and products. Our strategies include developing catalysts to transform CO2 and other greenhouse gases into fuels, chemicals, or functional materials.
Samples of our research
- Turning atmospheric CO2 into useful chemicals and fuels such as methanol, ethanol, or other chemicals by reacting it with carbon-neutral hydrogen generated using a renewable electricity source. Fuels synthesized by this process could be carbon-neutral, where the incorporated CO2 is reemitted upon fuel utilization, but atmospheric levels don’t increase. Incorporation of CO2-derived chemicals into long-lasting functional materials can also result in net reduction of atmospheric carbon to contribute to carbon-negative processes.
- Converting simple alkanes (such as methane, ethane, or propane) into useful chemicals. For instance, methane from landfills or vented at oil wells can be converted to hydrogen and syngas, or directly to methanol, a raw material for making many industrially important products. This can reduce emission of methane, a powerful greenhouse gas, into the atmosphere. Other alkanes can be catalytically reacted with carbon dioxide to make useful chemicals, thereby reducing the emission of multiple greenhouse gases.
Capabilities
- Catalysis reactivity and structure: Elucidating catalytically important properties over well-defined surfaces, powders, and nanostructures to advance fuel synthesis and energy conversion processes.
- Catalysis for alternative fuels production: Exploring the feasibility of producing value-added liquid products, such as aromatics and oxygenates, by developing catalytic processes in tandem with the CO2-assisted reforming and dehydrogenation of light alkanes.
- Nanostructure interfaces for catalysis: Identifying and characterizing the catalytically active sites of nanostructured surfaces and exploring how these are influenced or can be manipulated by variations in composition, particle size, morphology, and chemical environment.
- Structure and dynamics of applied nanomaterials: Understanding the relationships between microscopic and macroscopic properties of materials such as catalysts, electrocatalysts, solid oxide fuel cells, batteries, supercapacitors, phase change materials, and pyroelectrics.
- In situ and operando X-ray characterization of materials at the National Synchrotron Light Source II (NSLS-II)
- Material synthesis and characterization capabilities at the Center for Functional Nanomaterials (CFN), including the Interface Science and Catalysis Group: Capturing atomic-scale catalytic processes in real time and under reaction conditions to boost the reactivity of catalysts and enhance the capture of greenhouse gases.
- Advanced theory and computational modeling capabilities, including prediction and screening tools at the Computational Science Initiative
Net-Zero Carbon News
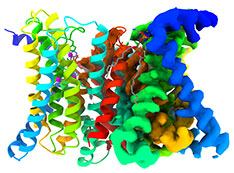
Zinc Transporter Has Built-in Self-regulating Sensor

Chemists Unravel Reaction Mechanism for Clean Energy Catalyst
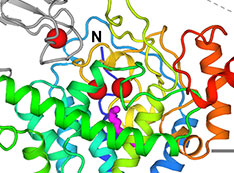
Structure of 'Oil-Eating' Enzyme Opens Door to Bioengineered Catalysts

DOE Announces $590 Million To Increase Bioenergy Research
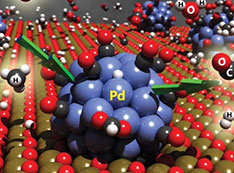
Novel Chemical Reaction Supports Carbon-Neutral Industrial Processes

Brookhaven Lab chemist Javier Concepcion and Lei Wang, a graduate student at Stony Brook University, devised a scheme for assembling light-absorbing molecules and water-splitting catalysts on a nanoparticle-coated electrode. The result: production of hydrogen gas fuel via artificial photosynthesis and a platform for testing different combos to further improve efficiency.
Catalysts for artificial photosynthesis, the ultimate clean energy source
Plants on earth harvest far more energy than is consumed by all human societies. Using energy from sunlight, they transform pure water and carbon dioxide into the sugars they use as fuel. Brookhaven chemists are working to mimic all or part of this process by developing catalysts that use sunlight to generate storable and transportable chemical fuels, ideally carbon-based fuels derived from CO2 in the atmosphere. In addition to transforming intermittent sunlight into an always-available energy source, this process would create a closed cycle of CO2 capture, conversion, storage, transport, and use, with the CO2 being emitted again when the fuel is burned. Such a closed cycle would recycle CO2 from the atmosphere rather than raising levels further (as burning fossil fuels does).

Brookhaven biochemist John Shanklin (center) with Hui Liu (left) and postdoc Zhiyang Zhai with tobacco plants used in a study to decipher a link between plants' sugar balance and oil production processes.
Rewiring plants to make fuels and bioproducts
Through photosynthesis, plants use sunlight and the atmospheric greenhouse gas CO2 to fuel their growth and development. Brookhaven biologists are working to understand the functions and regulation of plant genes involved in these biochemical pathways, particularly those that drive plants’ production of useful products like oils. Armed with this basic scientific knowledge, their goal is to “rewire” plants to produce abundant, sustainable quantities of key resources we now derive from petroleum-based chemicals—including oils that can be used as biofuels and raw materials for making products such as paints, lubricants, and plastics. Genomic and genetic studies will identify traits that enable such bioenergy/bio-product crops (e.g., “oilcane,” modified sugarcane that accumulates oil) to grow on marginal land, so they don’t compete for the fertile soil needed to grow the nation’s food supply.
Capabilities
- Expertise in genomics, biochemical genetics, computational modeling, and metabolic engineering at Brookhaven and at DOE’s Joint Genome Institute and Environmental Molecular Sciences Laboratory
- Access to world-leading structure and imaging capabilities at the National Synchrotron Light Source II and Center for Functional Nanomaterials




