Finding the Path of Least Resistance
A look at the past, present, and future of superconductivity research
July 22, 2009
Those who frequently use New York City’s subway system already know something about superconductivity – or at least the lack thereof.
Rumbling down rusted tracks designed around the turn of the 20th century, NYC trains produce a shuddering din of screeches and squeals instantly recognizable to local and tourist alike. Yet, while there is something to be said for city “character,” transit engineers may soon be able to quiet the subterranean grumblings.
Imagine an N-train that instead of rolling steel on steel over the Manhattan Bridge (with all the associated friction, heat and noise), gently floated over the river with hardly a sound. With superconductivity, it’s possible.
Since magnetic levitation was first proposed by BNL scientists James Powell and Gordon Danby in the 1960’s, governments and businesses around the world have pursued the development of “electrodynamic suspension,” a system in which a train would be suspended by invisible magnetic fields above a track, allowing it to travel more quickly, quietly and efficiently than any grounded counterpart. High-temperature superconductive materials, when they are fully understood, will be central to the success of this new technology.
As revolutionary as it promises to be, train levitation is only one potential use of superconductivity. Other advances may soon fundamentally alter the entire infrastructure by which the world moves and stores energy. Despite its rapid application, however, mastering the physics of the phenomenon has not been a smooth process at all. While scientists have known about and exploited superconductivity for almost as long as there have been trains in New York, recent developments demonstrate that there is much more to learn about the physical mechanisms underlying this fascinating phenomenon.
The sweet spot
Normal conductors – materials such as copper and aluminum – power everything from computers to can openers by providing a path for the flow of electrons, and thereby electricity, from power source to user. As the red glow of a toaster’s heating element demonstrates, however, these familiar systems operate at a cost. Almost every electrically conducting material in general use today exhibits some amount of “resistance,” or tendency to disrupt the flow of electrons, which results in lost energy, often in the form of heat. Toasters, of course, are designed to amplify this effect, but such inefficiency is a big problem for large distribution systems like the national power grid.
Superconductors, on the other hand, are not so stubborn. These unique materials, when situated in the right conditions, actually exhibit zero resistance, allowing electrons to move with complete freedom and efficiency. Start a current flowing in a loop of superconducting niobium, for example, and even with the battery removed, the current will continue to flow forever. Considering this amazing property, it is easy to see why engineers and scientists have big plans for superconductors.
But isolating and creating those “right conditions” is no easy task, since as it turns out, most materials require ultra-cold temperatures to activate their superconducting superpowers.
Freezing the desert
Heike Kamerlingh Onnes was first to definitively observe the phenomenon in 1911, building upon a half-century of scientific research into the properties of materials at ever-lowering temperatures. Using his own technique of helium liquefaction (a process itself only perfected three years prior) as a cooling agent, Onnes found that at precisely 4.19 Kelvin (about -452 degrees Fahrenheit), solid-state mercury loses all electrical resistance. His work resulted in a Nobel Prize in 1913.
Dozens of materials were found to be superconductors at various chilly “critical temperatures” in the decades following Onnes’ discovery, but it was not until 1957 that scientists established a workable theory for how this process operated. In that year, John Bardeen, Leon Cooper and Robert Schrieffer showed that superconductivity is really a macroscopic manifestation of electron behavior on the microscopic level of atoms.
To picture their idea (called BCS Theory), imagine the surface of a regular conducting material as the rough, uneven ground of the Arizona desert, and cast electrons as excitable figure skaters. If you apply energy (like an electrical current) to these skaters, they will attempt to trudge across the rocky terrain, but will often be tripped up (in the form of resistance) by the occasional tumbleweed or prairie dog. Also, due to the heat and their considerable egos (repulsive negative charges), they will tend to travel alone.
However, if you freeze the conductor landscape into a smooth sheet of ice, the electron-skaters behave very differently. Relieved of the maddening heat, the skaters will tend to overcome their natural revulsion for one another, forming pairs and then even acting as one large ensemble. Apply a current here, and the entire group will smoothly glide about the rink in unison with no resistance at all. Furthermore, they will continue their routine forever, since every pair would have to stop at once to avoid a group collision. Only with the return of heat will the pairs separate into their individual, fussy state.
A niche market
This harmonious ice dance is a fairly good description of what is happening in an ultra-cold superconductor. Heat energy causes the atomic lattices of solid materials to quiver unpredictably, making electron flow difficult, but super-cooled materials exhibit much less turbulence. Superconductors are unique because the strength associated with electron pairing causes any remaining lattice motion to be inconsequential.
Of course, the process of cooling materials to the required critical temperatures takes a lot of energy and ingenuity in itself – these magic numbers are usually mere degrees above absolute zero, after all. But if it is so difficult to create these conditions, what practical applications could these materials possibly have?
To be sure, for many years superconductivity played to a small crowd.
The most widespread use of the technology was in Magnetic Resonance Imaging (MRI) machines: medical devices used to create images akin to X-rays, but of soft tissues like the brain instead of only bone. MRI machines use a large, cylindrical superconducting magnet to apply a strong magnetic field to the hydrogen atoms in a patient’s body, causing them to “line up” in a predictable way that the scanner can then use to create an image. As in Onnes’ original experiment, these devices use a complicated and expensive cooling system of liquid helium to keep the magnet below its critical temperature.
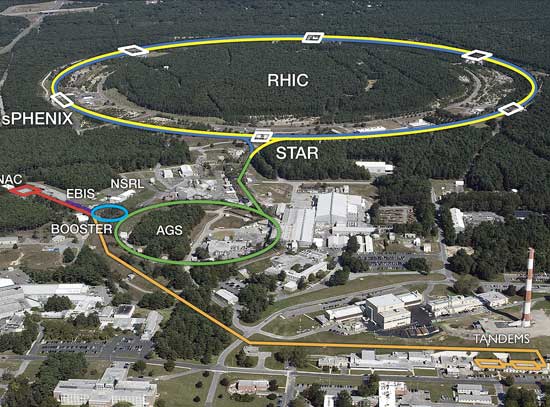
Aside from medical radiologists, the main user of superconductive science was the particle physics community. Large particle accelerators – machines like Brookhaven's Relativistic Heavy Ion Collider – use super-cooled superconducting magnets to direct atomic particles moving at incredibly high energies into intense collisions, recreating the conditions and exotic forms of matter present just after the Big Bang. Using sophisticated detectors, physicists are then able to analyze the debris in an effort to understand nature on the smallest scales.
Aside from these two applications, the prohibitive cost of cooling placed strict limits on the use of superconductors. Then, in the late 1980s, scientists made another great leap forward, finding that some special superconducting compounds are not so frigid after all.
A slow thaw
In 1986, Alex Müller and Georg Bednorz of IBM created a ceramic compound that became superconducting at the relatively high temperature of 30 Kelvin (-406 degrees Fahrenheit). Realizing that higher temperature superconductivity might be possible, scientists began rapidly testing other similar materials. Their search eventually resulted in the creation of complex materials that become superconducting at temperatures above the boiling point of liquid nitrogen, 77 Kelvin (-321 degrees Fahrenheit) in the 1990s. Since it is economically feasible to use the latter substance as a coolant, superconductivity could finally be used in commercial applications, including trains.
Currently, the material with the highest known temperature of superconductivity loses resistance at 138 Kelvin (-211 degrees Fahrenheit), and scientists are still searching for an “ideal” compound that would operate superconductively at room temperature, allowing for all kinds of novel applications. However, while industry professionals and scientists alike are thrilled with the developments in superconductivity, mysteries regarding the underlying physical mechanisms remain.
BCS Theory does not adequately explain superconducting phenomena at these higher temperatures. While it is unclear whether multiple explanations or an entirely new theory altogether is needed for superconductive science (even “string theory,” the vogue-but-controversial physics model which claims that all matter and fundamental forces arise from the vibrations of unimaginably small strands of energy, has joined the quest), scientists have recently made great progress.
New angles
At Brookhaven National Laboratory, two scientists, Seamus Davis and John Tranquada, recently received the 2009 Heike Kamerlingh Onnes Prize for Superconductivity Experiments (in conjunction with Aharon Kapitulnik of Stanford) for their work on high-temperature superconductors. Davis refined techniques of spectroscopic imaging with scanning tunneling microscopes that have been used to study cuprates, a special class of these materials.
Tranquada, on the other hand, pioneered a neutron scattering experiment that led to the discovery of “stripe phases” in cuprates. This characteristic, which has to do with electron arrangements, may be crucial in understanding superconductivity at high temperatures.
Another recent BNL finding shows that superconductivity at high energies may not only be related to the pairing tendency – it may also have to do with the range of angles in which the electron-skater pairs may execute their moves.
In results published in the June 26th issue of the journal Science, a Princeton team found that, in cuprates, optimal superconductivity occurs when electrons can pair up over the widest possible range of angles. Using a special scanning tunneling electron microscope, the team examined special cuprate crystals grown by Genda Gu and others at BNL in order to understand these materials on the atomic level. This discovery is important as it might explain the existence and value of the various critical temperatures – the point at which a material becomes superconducting.
Freeway to the future
The angle connection might also provide a means to pinpoint materials that could exhibit superconductivity at more quotidian temperatures.
Whether or not this mythical material exists, however, superconductors are sure to rapidly become an indispensible part of an increasingly technology-driven society. High-speed floating trains will soon carry passengers great distances in record times, in many cases faster than airplanes, and medical MRI machines could become much cheaper to build and operate, making life-saving diagnostic tests more widely available. Even the world’s power grids could one day convert to superconducting wires and transformers, making the production and consumption of electricity much more efficient and environmentally friendly.
While large-scale implementation of these and other applications of superconductivity are still years in the future, it is exciting to consider how much they will change so many aspects of daily life. With the loss of resistance, efficient energy and life-saving procedures – even a more peaceful commute – are only a few degrees away.
2009-1335 | INT/EXT | Newsroom