Blowing Nanobubbles in Perovskite Crystals
April 26, 2017
What is the scientific achievement?
CFN users and staff have directly observed how electron beams split water into hydrogen and oxygen using a perovskite oxide catalyst. This was found to not occur at the surface – as would be expected – but rather in the bulk, leading to bubbles in the catalyst that expand and contract with time.
Why does this achievement matter?
The interaction between water and oxides is critical for many applications, including energy storage, photocatalysis, surface wetting, and sensors. This work provides direct, nanoscale insight into the way an applied electrical bias can split water to yield hydrogen and oxygen, using a known, efficient photocatalyst.
What are the details?
 enlarge
enlarge
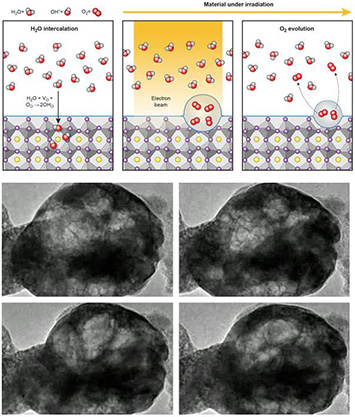
Top: Schematic illustrating water molecules entering the perovskite crystal, then breaking into hydrogen and water, leading to the formation of bubbles in the near surface region. Bottom: TEM images extracted from real time observations of the process.
CFN Capabilities:
These studies used the CFN environmental transmission electron microscope.
Publication Reference
B. Han,1 K. A. Stoerzinger,1 V. Tileli,2 A. D. Gamalski,3 E. A. Stach,3 Y. Shao-Horn,1 Nanoscale structural oscillations in perovskite oxides induced by oxygen evolution, Nat. Mater. 16, 121 (2017).
doi:10.1038/nmat4764
MIT News
Acknowledgement of Support
This work was supported in part by the MRSEC Program of the National Science Foundation under award number DMR-0819762 and the Skoltech-MIT Center for Electrochemical Energy Storage. The ETEM/EELS experiments were carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the US Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-SC0012704, which also supported A.D.G. and E.A.S.
2017-12217 | INT/EXT | Newsroom