Tripling the Energy Storage of Lithium-Ion Batteries
October 26, 2018
Collaborative effort between CFN Users from the University of Maryland, Oak Ridge National Laboratory, the U.S. Army Research Laboratory, and CFN staff members.
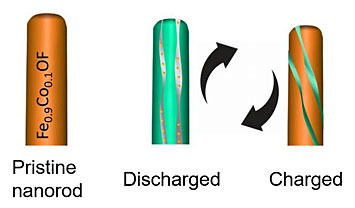
Illustration of (left) a pristine, doped nanorod (Fe0.9Co0.1OF), and (right) the nanorod structural evolution after charging and discharging cycles.
What is the scientific achievement?
CFN and University of Maryland scientists led a team of collaborators and users to develop and characterize doped nanorods made from iron fluoride (FeF3) by introducing cobalt and oxygen. The doped material stores a high energy density of ~1000 W·h kg-1. The nanorods are highly stable, retaining a high capacity of 420 mA·h g-1 after 1000 charge/discharge cycles and losing less than 0.03% of capacity with each cycle.
Why does this achievement matter?
Iron fluoride is an intercalation-conversion battery cathode material with a high theoretical energy density of 1922 W·h kg–1, but it suffers from poor cycle stability. The doped nanorods shown in this work improve reaction kinetics and cycling stability, which pave the way toward their use in next-generation lithium ion batteries.
What are the details?
Iron fluoride (FeF3), an intercalation-conversion cathode for lithium ion batteries, promises a high theoretical energy density of 1922 W·h kg-1. However, its poor electrochemical reversibility due to repeated breaking and reformation of metal fluoride bonds poses a grand challenge for practical applications. This work reports both a high reversibility over 1000 cycles and a high capacity of 420 mA·h g-1 can be realized by concerted doping of cobalt and oxygen into FeF3. In the doped nanorods, an energy density of ~1000 W·h kg-1 with a decay rate of 0.03% per cycle is achieved. The anion’s and cation’s co-substitutions thermodynamically reduce conversion reaction potential and shift the reaction from less-reversible intercalation-conversion reaction in FeF3 to a highly reversible intercalation-extrusion reaction in the doped material. The co-substitution strategy to tune the thermodynamic features of the reactions could be extended to other high energy conversion materials for improved performance.
CFN Capabilities:
The CFN Electron Microscopy facility and NSLS-II XPD beamline were used for multi-modal, in-situ characterization by electron beams and X-rays.
Publication Reference
X. Fan, E. Hu, X. Ji, Y. Zhu, F. Han, S. Hwang, J. Liu, S. Bak, Z. Ma, T. Gao, S.-C. Liou, J. Bai, X.-Q. Yang, Y. Mo, K. Xu, D. Su, C. Wang, High energy-density and reversibility of iron fluoride cathode enabled via an intercalation-extrusion reaction, Nature Communications 9, 2324 (2018).
DOI: 10.1038/s41467-018-04476-2
Tripling the Energy Storage of Lithium-Ion Batteries
Acknowledgement of Support
X.F. and C.W. acknowledge the financial support from Army Research Lab under Award Number W911NF1420031. Daikin America provided the high purity fluorinated solvents. The PDF data were collected at XPD beamline (28ID-2) of NSLS-II, and the electron microscopy analysis was carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory (BNL), which is supported by the DOE, Office of Basic Energy Sciences, under contract DE-SC0012704. The PDF studies at BNL were supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the U.S. Department of Energy through the Advanced Battery Materials Research (BMR) Program, including Battery500 Consortium under contract DE-SC0012704. C.W. and K.X. also acknowledge the support of EERE of USDOE through Battery500 Consortium Seeding project under contract DE-EE0008200. The authors acknowledge the University of Maryland supercomputing resources (http://hpcc.umd.edu) made available for conducting DFT computations in this paper. We also appreciate Dr. Qingping Meng at BNL, and Dr. Nancy J. Dudney at Oak Ridge National Laboratory (ORNL) for the constructive discussions.
2018-14398 | INT/EXT | Newsroom