The Science Behind the Shot: Biotech Tools Developed at Brookhaven Lab Fundamental to Making COVID-19 Vaccines
Enzymes and promoters based on 'T7 expression system' crank out copious quantities of mRNA for vaccinating people around the world
April 13, 2021
 enlarge
enlarge
F. William Studier, Senior Scientist Emeritus at Brookhaven National Laboratory, in 2004. In the 1980s, Studier and a team of collaborators deciphered key details of the T7 bacteriophage and turned what they learned into a system to produce copious amounts of desired proteins. The production of today's mRNA COVID-19 vaccines relies on these foundational discoveries.
You’ve probably heard that the first two vaccines approved for battling COVID-19 in the United States use a relatively new approach—injections of simple packets containing mRNA, a genetic material that instructs our cells to make coronavirus spike proteins. But the technology for generating sufficient amounts of those mRNA packets dates back to the 1980s, when F. William Studier, then a senior biophysicist at the U.S. Department of Energy’s Brookhaven National Laboratory, developed a way to harness the molecular machinery of a very different virus.
“The fact that scientific knowledge and tools developed decades ago are now being used to produce today’s lifesaving mRNA vaccines for COVID-19 is a great example of how the Department of Energy’s long-term investments in fundamental research at our National Laboratories can improve American lives today and into the future,” said Dr. Steve Binkley, Acting Director of DOE’s Office of Science.
In the 1980s, Studier, the late John Dunn, and their group in Brookhaven Lab’s Biology Department completed sequencing and annotating the genome of the T7 bacteriophage. T7 is a virus that infects E. coli bacteria and commandeers those cells to make copies of the virus. Having the complete genome sequence helped the scientists understand how T7 genes and other elements work together to reproduce many copies of the virus.
Shortly thereafter, Studier and his team found a way to direct T7’s prolific copying capability toward making things other than more T7s. They cloned the T7 RNA polymerase—the enzyme that transcribes DNA genes into messenger RNA (mRNA), which instructs cells which amino acids to link up to build a particular protein. The team then used this T7 RNA polymerase along with a powerful T7 promoter (a genetic element that serves as a “start” signal for gene transcription) to produce large amounts of RNA from almost any gene. These RNAs could be used directly, such as in mRNA-based vaccines, or delivered to ribosomes (cells’ protein-making factories) to be translated into proteins, as in the T7 expression system.
“Scientists around the world have used Bill’s ‘T7 expression system’ to make large quantities of proteins or RNA of interest for the past four decades,” said Venki Ramakrishnan, a Nobel-Prize-winning structural biologist from the Medical Research Council Laboratory of Molecular Biology, in Cambridge, UK. “The new mRNA COVID-19 vaccines use precisely this system,” he said.
At manufacturing plants run by Pfizer/BioNTech and Moderna, T7-derived promoters and enzymes crank out kilograms of spike-protein mRNA at a time—leaving our own cells to do the protein-making part after a dose of instructions is injected into our arms.
“Bill’s fundamental work made the lifesaving mRNA vaccines possible,” Ramakrishnan said.
Back to basics
 enlarge
enlarge
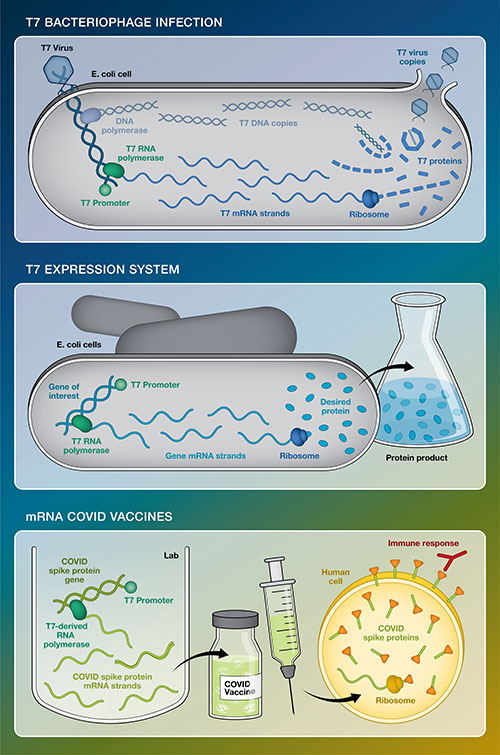
This simplified graphic compares T7 bacteriophage infection with the T7 expression system developed at Brookhaven Lab in the 1980s and the use of T7 genetic elements to make today's mRNA COVID-19 vaccines. All three use the T7 promoter as a signal to start gene transcription and T7-derived RNA polymerase to transcribe the DNA code into mRNA instructions that "tell" ribosomes how to build proteins. In T7 infection, the product is new viruses (protein coats encapsulating copies of T7 DNA and other viral proteins). The expression system produces one particular desired protein. For the vaccine, the mRNAs are injected into our arms where our ribosomes use them to make COVID-19 spike proteins. Those virus-free spikes train our immune system to be ready if we have to battle the real virus.
William Studier had no visions of making vaccines when he began his fundamental research on the T7 virus as a graduate student in biophysics at the California Institute of Technology, nor while continuing this research after joining Brookhaven Lab.
“T7 was not a well-studied bacteriophage when I came to Brookhaven in 1964,” he said. “I was using it to study properties of DNA and decided also to study its molecular genetics and physiology. My goal, of course, was to understand as much as possible about T7 and how it works.”
Cloning the gene for T7 RNA polymerase in 1983 was particularly difficult, Studier noted.
“Others had tried and failed. Companies had asked me if we had it,” he said. “We had the DNA sequence then and I thought that I understood why the cloning had failed and how to remedy the problem.”
Studier worked with Parichehre Davanloo, then a postdoctoral fellow, and Alan Rosenberg, a senior lab member, to tackle the challenge. John Dunn purified the T7 RNA polymerase and showed that it produces large amounts of RNA. Barbara Moffatt, then a graduate student, worked with Studier to turn these discoveries into the T7 expression system.
“The Brookhaven Lab patent attorney knew what we were doing and we filed what I think was the first patent at Brookhaven under the Bayh-Dole Act”—a law passed by Congress in 1980 that allowed institutions and grant recipients to patent and license rights to inventions stemming from government-funded research, Studier explained.
The rest is history. The T7 expression system went on to become Brookhaven Lab’s most successful technology, with research labs around the world using it and hundreds of companies licensing the technology to make products. And although the patents have now expired, T7 is still the go-to system for biochemists everywhere.
“T7 RNA polymerase can synthesize large amounts of RNA from any DNA adjacent to a strong T7 promoter. I don’t know whether there is anything comparable,” Studier said.
John Shanklin, chair of Brookhaven Lab’s Biology Department, agrees. “The T7 polymerase and promoter are so efficient, they make it possible to produce enough mRNA for millions of vaccine doses at a time. Bill’s discoveries played a huge role in making it possible to rapidly scale up production to protect people around the world from COVID-19.”
Two of those recently vaccinated people are Bill Studier and his wife Sue.
“I had wondered casually if T7 RNA polymerase might be involved in making the RNA vaccines,” mused Studier, now a senior biophysicist Emeritus. “Basic research is almost always useful, and I’m pleased that my work has been helpful in obtaining powerful vaccines against this pandemic.”
Slideshow of historical images of William Studier, taken in 1970, 1972 (with Polly Winston), 1978, and 1984 (with John Dunn). Hover over image to reveal slideshow controls.
F. William Studier earned a B.S. in biophysics from Yale in 1958, followed by a Ph.D. from the California Institute of Technology in 1963. He worked as a postdoctoral fellow in the Department of Biochemistry at Stanford University School of Medicine, and then joined Brookhaven Lab’s Biology Department in 1964 as an assistant biophysicist. Over the years, Studier rose through the department’s ranks, receiving tenure in 1971 and becoming a tenured senior biophysicist in 1974. He served as chair of the Biology Department from 1990 to 1999 and then returned to research. He also served as an Adjunct Professor of Biochemistry at Stony Brook University. His achievements have been recognized by election to the American Academy of Arts and Sciences in 1990, the National Academy of Sciences in 1992, and as a Fellow of the American Association for the Advancement of Science in 2007. In 2015 he was named Senior Scientist Emeritus at Brookhaven Lab, and was elected as a Fellow of the National Academy of Inventors in 2018. He holds 15 patents of which 9 have been licensed and commercialized, including those on the T7 system.
Studier’s research at Brookhaven Lab was supported by the DOE Office of Science (BER).
Brookhaven National Laboratory is supported by the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://www.energy.gov/science/.
Follow @BrookhavenLab on Twitter or find us on Facebook.
2021-18806 | INT/EXT | Newsroom