Producing Valuable Chemicals from Waste Carbon Dioxide
September 30, 2016
What Is The Scientific Achievement?
A simple method was used to fabricate copper surfaces that efficiently convert carbon dioxide (CO2) into useful industrial chemicals. Oxidizing the surface of copper with a plasma creates a nanostructured surface that becomes highly selective to the formation of ethylene (C2H4). The ethylene production was found to depend on both the surface roughness and the copper species that exist on the surface due to the plasma pre-treatment. This insight could lead to the production of cost-effective routes toward the synthesis of C2H4 from CO2.
Why Does This Matter?
Carbon dioxide is a greenhouse gas that is produced when fossil fuels are burned. This work demonstrated a simple, low-cost route to the the conversion of carbon dioxide into ethylene. Ethylene, a widely-employed chemical feedstock, is used in the production of polyethylene plastics, among other materials. The ability to take a potentially harmful waste product and to convert it into a useful chemical could be a game-changing, cost effective approach toward CO2 capture and pollution remediation.
What Are The Details?
 enlarge
enlarge
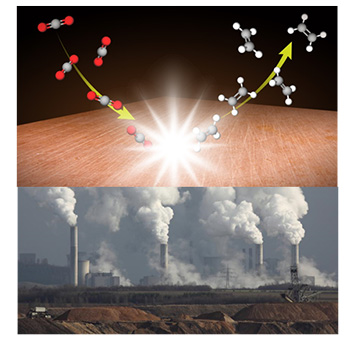
Top) A graphical representation of the CO2 to C2H4 reaction on copper. Bottom) A photo of waste CO2 being emitted from a coal-burning electricity plant. Efficient conversion of waste carbon dioxide into useful ethylene gas has great potential for effective pollution remediation, which could help mitigate global warming.
CFN Capabilities: Site-specific sample preparation for transmission electron microscopy was performed in CFN's Electron Microscopy facility. CFN Staff also contributed to the data analysis and interpretation.
Publication Reference
Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene
Hemma Mistry1,2, Ana Sofia Varela3, Cecile S. Bonifacio4, Ioannis Zegkinoglou2, Ilya Sinev2, Yong-Wook Choi2, Kim Kisslinger5, Eric A. Stach5, Judith C. Yang4, Peter Strasser3 & Beatriz Roldan Cuenya2
1 Department of Physics, University of Central Florida, Orlando, Florida 32816, USA.
2 Department of Physics, Ruhr-University Bochum, 44780 Bochum, Germany.
3 Department of Chemistry, Chemical Engineering Division, Technical University Berlin, 10623 Berlin, Germany.
4 Chemical and Petroleum Engineering and Physics, University of Pittsburgh, Pittsburgh, Pennsylvania 15261, USA.
5 Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, New York 11973, USA.
Nature Communications 7, 12123 (2016)
Acknowledgement of Support:
The authors acknowledge ?nancial assistance from the NSF under Grant No. DMR-0907483. Research was carried out in part at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, O?ce of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886.
2016-6616 | INT/EXT | Newsroom