Revealing the Growth Behavior of New Nanoparticle Catalysts
Platinum nickel nanoparticles are a promising catalyst in hydrogen fuel cells if their growth can be controlled
November 30, 2018
 enlarge
enlarge
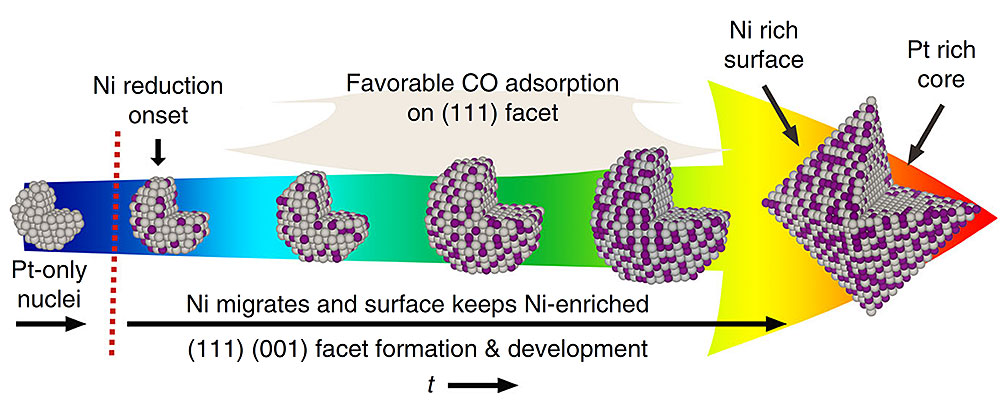
The nanoparticles grow from a Pt-only core into Pt3Ni octahedral with a Pt (in grey) core and a Ni-rich (Ni in purple) surface. Image courtesy of X. Shen, Nat Comm 9:4485 (2018)
The Science
Scientists discovered that carbon monoxide drives the growth of a new platinum nickel nanoparticle catalyst.
The Impact
Understanding the growth pathway of faceted alloy nanoparticles at the atomic level is crucial to controlling their properties as a future catalyst for hydrogen fuel cells.
Summary
Hydrogen fuel cells are a promising technology for producing clean and renewable energy, but the cost and activity of their cathode materials is a major challenge for commercialization. To tackle this challenge, scientists are searching for new catalysts that are not made of pure platinum. One promising option is platinum nickel (Pt-Ni) nanoparticles. Even though the catalytic capabilities of Pt-Ni nanoparticles are known, the process under which they grow into specific shapes is not well understood.
In this study, scientists used a combination of techniques, including scanning transmission electron microscopy and x-ray spectroscopy, to measure the growth of Pt-Ni nanoparticles in real time. The team worked with scientists from the In situ and Operando Soft X-ray Spectroscopy (IOS) beamline of the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory.
After their measurements, they used a first principle computer simulation to analyze and verify their data. Based on their analysis and the combined data, they determined a clear pathway for the nanoparticle’s growth and facet formation.
The researchers discovered that carbon monoxide molecules serve as a facet formation modulator and causes the nanoparticle’s shape to evolve from a spherical cluster to the desired octahedron. The scientists were also able to show that the nanoparticle’s spherical core is only made of Pt and slowly adds the Ni during the growth process. The Ni mainly stays in the new layers that are close to the surface of the newly formed nanoparticle.
This fundamental work highlights the significant role of segregated nickel in forming the octahedral-shaped catalyst and offers new insight into shape control of catalyst nanoparticles.
Related Links
Feature Story: “Illuminating Nanoparticle Growth with X-rays”
Contact
Zhenmeng Peng
The University of Akron
zpeng@uakron.edu
Publications
X. Shen, C. Zhang, S. Zhang, S. Dai, G. Zhang, M. Ge, Y. Pan, S. M. Sharkey, G. W. Graham, A. Hunt, I. Waluyo, J. T. Miller, X. Pan, Z. Peng, Deconvolution of octahedral Pt3Ni nanoparticle growth pathway from in situ characterizations. Nature Communnications 9:4485 (2018). DOI: 10.1038/s41467-018-06900-z
Funding
This work was supported by National Science Foundation (grants 1665265, DMR-1506535 and CBET-1159240) and the Center for Innovative and Strategic Transformation of Alkane Resources (CISTAR) under Cooperative Agreement No. EEC-1647722. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Basic Energy Sciences [grant number DE-AC02-06CH11357]. MRCAT operations, beamline 10-BM, are supported by the Department of Energy and the MRCAT member institutions. This research used resources of the 23-ID-2 beamline of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. This research is also partially supported by Laboratory Directed Research and Development (LDRD15-037) program at Brookhaven National Laboratory.
2018-17586 | INT/EXT | Newsroom