New Approach for Ammonia Production
Scientists show first proof of principle for improved ammonia production to meet today's global food needs
January 31, 2021
 enlarge
enlarge
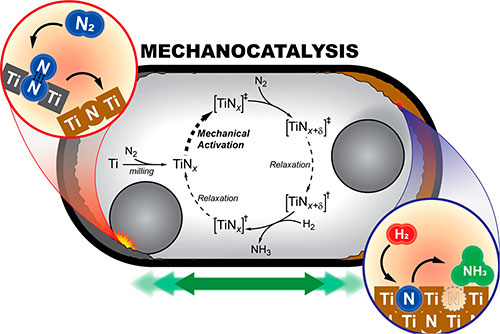
Mechanocatalytic ammonia synthesis by a titanium catalyst, in a ball mill, under nitrogen and hydrogen gas flow. Image credit: Andrew W. Tricker, et. Al. ACS Energy& Letters 5 (11), 3362-3367 (2020).
The Science
Scientists show the first proof of principle for ammonia production through ball milling using a titanium nitride catalyst under nitrogen and hydrogen gas flow at room temperature.
The Impact
To meet today’s global food needs, fertilizers based on ammonia are key; this work offers an environmentally friendly and energy-efficient way to synthesize ammonia under ambient conditions.
Summary
To meet today’s global food needs, fertilizers based on ammonia are key. However, the synthesis process of ammonia is burdened by two competing factors: breaking the bond between two nitrogen atoms requires a significant amount of energy or heat, while ammonia formation is more efficient at lower temperatures. The current industrial process happens under harsh conditions and accounts for ∼2% of global energy consumption and ∼1% of global CO2 emissions. Therefore, scientists are searching for new industrial processes that operate under mild conditions fueled by renewable energy sources to decarbonize the ammonia industry.
In this work, a team of scientists synthesized ammonia, for the first time, under ambient conditions. They used a mechanocatalytic reaction under continuous gas flow in a titanium ball mill. In this reaction, ammonia was synthesized through the help of titanium nitrate, which formed through the milling of titanium powder (Ti) in a vibratory ball mill under nitrogen and hydrogen gas flow.
The scientists used multiple methods, including x-ray absorption spectroscopy at the Inner-Shell Spectroscopy (ISS) Beamline at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory, to confirm the formation of titanium nitride from titanium and N2.
Based on their experiments, the scientists showed that the newly formed titanium nitride is catalytically active and furthers the reaction from nitrogen and hydrogen to form ammonia. They also found that the catalytic cycle is rate limited by the nitride regeneration, thus a more stable nitride is expected to yield higher ammonia rates.
This new process opens the pathway to a novel approach for industrial ammonia production and could potentially be used for other catalytic reactions with similar competing factors.
Download the research summary slide
Contact
Carsten Sievers
Georgia Institute of Technology
carsten.sievers@chbe.gatech.edu
Publication
A. W. Tricker, K. L. Hebisch, M. Buchmann, Y.-H. Liu, M. Rose, E. Stavitski, A. J. Medford, M. C. Hatzell, C. Sievers. Mechanocatalytic Ammonia Synthesis over TiN in Transient Microenvironments. ACS Energy Letters 5 (11), 3362-3367 (2020). DOI: 10.1021/acsenergylett.0c01895
Funding
A.W.T. acknowledges support from a PSE fellowship from the Renewable Bioproducts Institute at the Georgia Institute of Technology. K.L.H. thanks the Bundesministerium fu¨r Bildung und Forschung (German Federal Ministry of Education and Research) and BASF SE for their support by the Deutschlandstipendium. M.C.H. and Y.-H.L. acknowledge that this material is based upon work supported by the National Science Foundation under Grant No. 1846611. M.R. and M.B. acknowledge financial support by the German Research Foundation (Grant No. RO 4757/5-1). This research used resources of the beamline 8-ID ISS (Inner Shell Spectroscopy) in the National Synchrotron Light Source II and the Center for Functional Nanomaterials, which are U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. The XRD work was performed in part at the Materials Characterization Facility (MCF) at Georgia Institute of Technology. The MCF is jointly supported by the GT Institute for Materials (IMat) and the Institute for Electronics and Nanotechnology (IEN), which is a member of the National Nanotechnology Coordinated Infrastructure supported by the National Science Foundation (Grant ECCS-1542174).
2021-18846 | INT/EXT | Newsroom