Fast Cooldown for Making Battery Materials
To develop better battery materials, scientists take a close look at how the materials should cool down during synthesis
May 31, 2020
 enlarge
enlarge
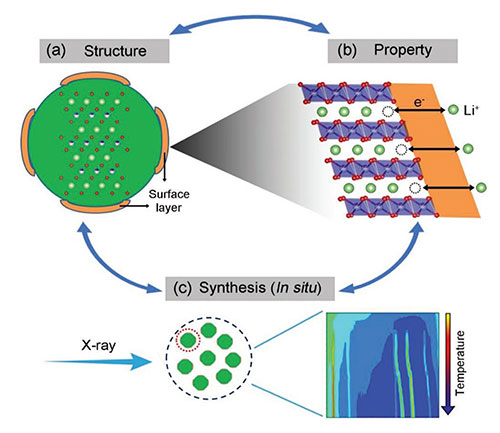
The "closed" loop approach for the rational design and synthesis of high-Ni layered oxides using multimodal x-ray studies to understand the property-structure connection. Image credit: M.-J. Zhang et al. Adv. Energy Mater. 9, 1901915 (2019)
The Science
Scientists revealed how the cooling rate during synthesis impacts the chemical properties of high-nickel (high-Ni) layered oxides used as potential battery cathode materials.
The Impact
Li-ion batteries (LIBs) are increasingly used to power electric vehicles, but a challenge for their mass adoption is low capacity; this study provides design guidance on synthesizing high-Ni layered cathodes.
Summary
From cellphones to electric vehicles, we use lithium-ion batteries (LIBs) in a variety of applications. Moreover, the demand for larger LIBs in applications that require more power.
However, LIBs often fall short for practical mass adoption due to their low lithium-storage (Li-storage) capacity, which is largely constrained by cathodes. Therefore, scientists have searched for improved cathode candidates and transition metal (TM) layered oxides with a hexagonal structure, which showed great potential. In particular, high-nickel (high-Ni) layered oxides show very high theoretical capacity, however, these cathodes show low stability over many cycles and their safety is often compromised due to the surface-related issues.
In this work, a team of scientists synthesized a representative of high-nickel (high-Ni) layered oxides, NMC71515, and investigated how the synthesis conditions, particularly the cooling rate, affect the structural evolution, not only in the bulk, but also at the surface. In order to effectively follow the changes in the material during the different cooling conditions, the team used a variety of research techniques that they could apply in situ, which means while both the synthesis and cooling process was happening.
Among these techniques, the team used the X-ray Powder Diffraction (XPD) beamline at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory, to investigate the chemical properties of the material during the cooldown and the behavior during quenching. Once the samples had cooled down, the team used the Full-Field X-ray Imaging (FXI) beamline to take a closer look at the surface condition of the cathode particles and how they differed from the bulk depending on how the sample had been cooled down.
By combining the results from in situ x-ray diffraction, local x-ray imaging studies with their additional structural and electrochemical analysis, they concluded that the surface reconstruction is cooling rate dependent and affects the performance. The performance can be improved by suppressing the reconstruction through quenching. This work offers a new path to rationally design Ni-high layered oxides for use as battery cathode materials with high capacity.
Download the research summary slide
Contact
Feng Pan
Peking University
panfeng@pkusz.edu.cn
Feng Wang
Brookhaven National Laboratory
fwang@bnl.gov
Jianming Bai
National Synchrotron Light Source II
Brookhaven National Laboratory
jmbai@bnl.gov
Publication
Zhang, M.-J., Hu, X., Li, M., Duan, Y., Yang, L., Yin, C., Ge, M., Xiao, X., Lee, W.-K., Ko, J. Y. P., Amine, K., Chen, Z., Zhu, Y., Dooryhee, E., Bai, J., Pan, F., Wang, F., Cooling Induced Surface Reconstruction during Synthesis of High-Ni Layered Oxides. Adv. Energy Mater. 9, 1901915 (2019). DOI: 10.1002/aenm.201901915
Funding
This work was supported by the U.S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office, Contract No. DE-SC0012704, the Guangdong innovative team program (2013N080), Guangdong Key-lab Project (No. 2017B0303010130), the peacock plan (KYPT20141016105435850), Shenzhen Science and Technology Research Grant (ZDSYS201707281026184), Chinese postdoctoral science foundation (2015M570882, 2015M570894). This research used 28-ID-2 (XPD) and 18-ID (FXI) beamlines at the National Synchrotron Light Source II, a U. S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. The electron microscopy work at BNL is supported by the U.S. Department of Energy, Office of Basic Energy Science, Division of Materials Science and Engineering, under Contract No. DE-SC0012704. Research at the Argonne National Laboratory was funded by the US Department of Energy, Vehicle Technologies Office under contract no. DE-AC02-06CH11357.
2020-18945 | INT/EXT | Newsroom