Improving a Natural Gas Conversion Catalyst
Scientists strive to improve the performance of catalysts for turning greenhouse gases into viable chemicals
May 31, 2020
 enlarge
enlarge
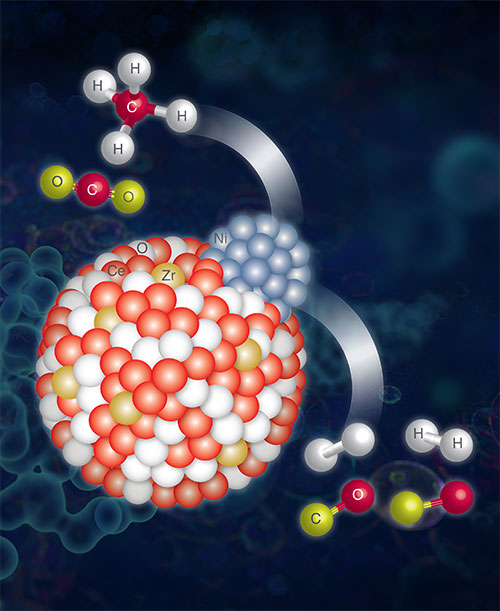
An artist's impression of the Zr-doped Ni-supported ceria (CeO2) catalyst performing a DRM reaction. Image credit: F. Zhang et al. ACS Catalysis 10 (5), 3274-3284 (2020).
The Science
Scientists revealed how zirconium (Zr) doping improved the catalytic performance on nickel (Ni) supported ceria (CeO2) catalysts for hydrocarbon conversion.
The Impact
Converting greenhouse gases, such as CH4 and CO2, into useful intermediates or products offers green solutions for commercial fuel processing; this study revealed details about a mechanism to enhance catalytic performance.
Summary
Natural gas has transformed the fossil fuel market. It is primarily composed of methane, which is a potent greenhouse gas. At present, it is important to find efficient ways to enable sustainable use as an alternative to other fossil fuels, such as coal or petroleum. Therefore, researchers are searching for viable solutions for sustainable, commercial, natural gas processing, which should also be a practical solution to the irreversible and harmful impact of global greenhouse gas emissions.
In this work, researchers studied a catalyst composed of nickel-zirconia-ceria, which enhanced the dry reforming of the methane reaction (DRM: CH4 + CO2 -> 2CO + 2H2) that changes both CH4 and CO2 into useful intermediates, or products for further usage, instead of releasing those greenhouses into the atmosphere. Even though ceria, a viable catalyst for this reaction, is in its normal state it is not very cost-effective since activation and conversion need to be handled in different steps. To address this problem the scientists added zirconium (Zr) to the ceria structure to enhance the efficiency and selectivity of the catalysts and therefore, reduce the energy cost.
The researchers used various characterization techniques to understand the catalyst’s behavior during difficult DRM reaction conditions. These techniques included in situ x-ray studies at the Pair Distribution Function (PDF) beamline of the National Synchrotron Light Source II (NSLS-II) and beamlines 17BM and 21ID at the Advanced Photon Source (APS) and electron microscopy studies at the Center for Functional Nanomaterials (CFN). All three facilities are U.S. Department of Energy (DOE) Office of Science User Facility with both NSLS-II and CFN being located at DOE’s Brookhaven National Laboratory, while the APS is located at DOE’s Argonne National Laboratory.
Through these studies, the scientists found that by introducing Zr into the ceria catalyst, the catalytic performance, including conversion, reaction rate, and H2 selectivity, was significantly enhanced. They were able to reveal details about the reaction mechanism and showed that Zr prevented nickel migration to the surface of the catalyst and therefore, preserved the active surface area of the catalyst.
This study offers a more detailed understanding of catalytic reaction mechanism and a pathway to improve both CH4 and CO2 conversion.
Download the research summary slide
Contact
Sanjaya D. Senanayake
Brookhaven National Laboratory
ssenanay@bnl.gov
Petar Djinovic
National Institute of Chemistry
petar.djinovic@ki.si
Publication
F. Zhang, Z. Liu, X. Chen, N. Rui, L. E. Betancourt, L. Lin, W. Xu, C.-j. Sun, A. M. M. Abeykoon, J. A. Rodriguez, J. Teržan, K. Lorber, P. Djinovic, S. D. Senanayake. Effects of Zr Doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 Catalysts: In Situ Studies with XRD, XAFS, and AP-XPS. ACS Catalysis 10 (5), 3274-3284 (2020). DOI: 10.1021/acscatal.9b04451
Funding
The work carried out at Brookhaven National Laboratory was supported by the US Department of Energy under contract no. DE-SC0012704. S.D.S. is supported by a US Department of Energy Early Career Award. The PDF measurement used resources 28ID-1 of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. This research also used resources of the Center for Functional Nanomaterials, specifically the electron microscopy facilities, which is a U.S. DOE Office of Science Facility at Brookhaven National Laboratory under contract no. DE-SC0012704. The XRD and XAFS experiments used resources of the Advanced Photon Source Beamline 17BM (XRD) and 20ID (XAFS) at Argonne National Laboratory, which is an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science and was supported by the U.S. DOE under contract no. DE-AC02-06CH11357 and the Canadian Light Source and its funding partners. J.T., K.L., and P.D. are funded by Slovenian national research agency (ARRS) through research program P2-150 and projects J2-1726 and BI-US/18-20-004.
2020-18946 | INT/EXT | Newsroom