Recyclable Reagent and Sunlight Convert Carbon Monoxide into Methanol
Room-temperature, ambient-pressure conversion reaction for carbon monoxide could be part of a larger cascade strategy for efficiently turning atmospheric carbon dioxide (CO2) into liquid fuel
March 20, 2024
 enlarge
enlarge
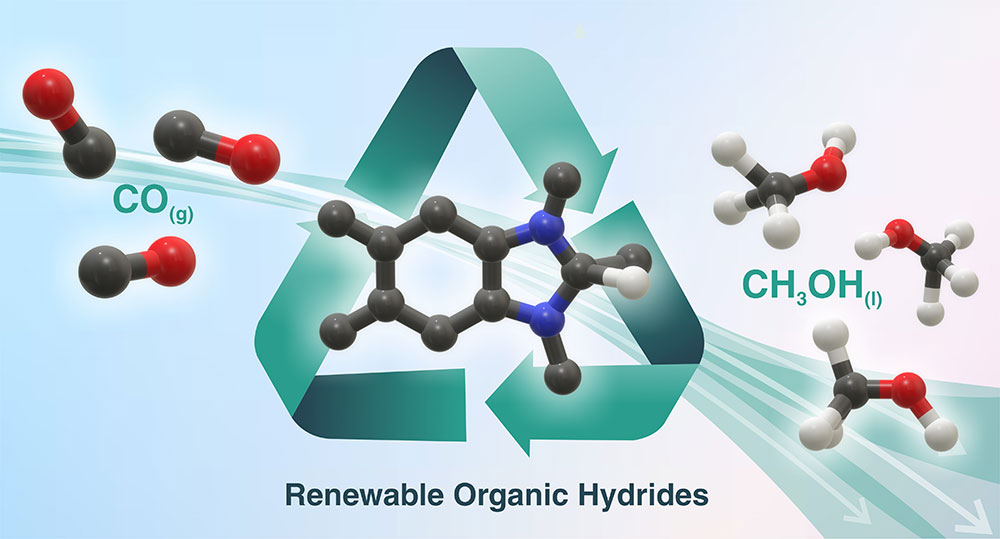
Brookhaven National Laboratory and University of North Carolina Chapel Hill researchers have identified renewable organic hydrides that can efficiently convert carbon monoxide (CO) to methanol (CH3OH). These reagents could be part of a cascade strategy for converting atmospheric carbon dioxide (CO2) into easily transportable/storable liquid fuel. (Andressa Muller/Brookhaven National Laboratory)
UPTON, NY — Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and the University of North Carolina Chapel Hill (UNC) have demonstrated the selective conversion of carbon dioxide (CO2) into methanol using a cascade reaction strategy. The two-part process is powered by sunlight, occurs at room temperature and at ambient pressure, and employs a recyclable organic reagent that’s similar to a catalyst found in natural photosynthesis.
“Our approach is an important step toward finding an efficient way to convert CO2, a potent greenhouse gas that poses a significant challenge for humanity, into an easily storable and transportable liquid fuel,” said Brookhaven Lab Senior Chemist Javier Concepcion, a lead author on the study.
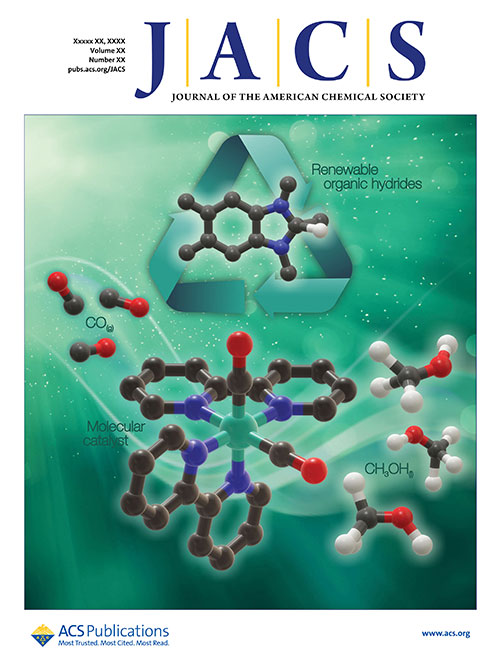
The research was conducted as part of the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), an Energy Innovation Hub based at UNC and funded by the DOE Office of Science. The study is published as the "front cover" article in the Journal of the American Chemical Society.
 enlarge
enlarge
Brookhaven Lab members of the research team (left to right): Andressa Muller, David Grills, Zahid Ertem, Dmitry Polyansky, and Javier Concepcion, all of the Lab's Chemistry Division. (Kevin Coughlin/Brookhaven National Laboratory)
The room-temperature conversion of CO2 into liquid fuels has been a decades-long quest. Such strategies could help achieve carbon-neutral energy cycles, particularly if the conversion is powered by sunlight. The carbon emitted as CO2 by burning single-carbon fuel molecules such as methanol could essentially be recycled into making new fuel without adding any new carbon to the atmosphere.
Methanol (CH3OH) is a particularly attractive target because it is a liquid that can be easily transported and stored. In addition to its usefulness as a fuel, methanol serves as a key feedstock in the chemical industry for making more complex molecules. Also, because methanol contains just one carbon atom, like CO2, it circumvents the need for making carbon-carbon bonds, which require energy-intensive processes.
However, key steps involved in the reactions required to selectively and efficiently generate solar liquid fuels like methanol remain poorly understood.
“Converting CO2 to methanol is very difficult to achieve in a single step. It is energetically akin to climbing a very tall mountain,” Concepcion said. “Even if the valley on the other side is at lower altitude, getting there requires a lot of energy input.”
 enlarge
enlarge
University of North Carolina Chapel Hill members of the research team (left to right): CHASE Director Gerald Meyer, Renato Sampaio, and Jake Sirlin. (UNC)
Instead of trying to tackle the challenge in a single “climb,” the Brookhaven/UNC team used a cascade, or multi-step, strategy that goes through several intermediates that are easier to reach.
“Imagine climbing several smaller mountains instead of a big one — and doing so through several valleys,” Concepion said.
The valleys represent reaction intermediates. But even reaching those valleys can be difficult, requiring the stepwise exchange of electrons and protons among various molecules. To lower the energy requirements of these exchanges, chemists use molecules called catalysts.
“Catalysts enable reaching the next valley through ‘tunnels’ that require less energy than climbing over the mountain,” Concepcion said.
 enlarge
enlarge
This research was selected as the cover article in the Journal of the American Chemical Society. (Andressa Muller/Brookhaven National Laboratory)
For this study, the team explored reactions employing a class of catalysts called dihydrobenzimidazoles. These are organic hydrides — molecules that have two extra electrons and a proton to “donate” to other molecules. They are inexpensive, their properties can be easily manipulated, and previous studies have shown that they can be recycled, a requirement for a catalytic process.
These molecules are similar in structure and function to organic cofactors responsible for carrying and delivering energy in the form of electrons and protons during natural photosynthesis.
“Photosynthesis itself is a cascade of many reaction steps that convert atmospheric CO2, water, and light energy into chemical energy in the form of carbohydrates — namely sugars — that can later be metabolized to fuel the activity of living organisms. Our approach of using biomimetic organic hydrides to catalyze methanol as a liquid fuel can therefore be viewed as an artificial approach to photosynthesis,” said UNC co-lead author Renato Sampaio.
In the study, the chemists broke the conversion of CO2 into methanol into two steps: photochemical reduction of CO2 to carbon monoxide (CO), followed by sequential hydride transfers from dihydrobenzimidazoles to convert the CO into methanol.
Their work describes the details of the second step, as the reaction proceeds through a series of intermediates, including a ruthenium-bound carbon monoxide (Ru-CO2+) group, a ruthenium formyl (Ru-CHO+) moiety, a ruthenium hydroxymethyl (Ru-CH2OH+) group, and finally, light-induced methanol release.
 enlarge
enlarge
First author Andressa Muller measuring reaction kinetics using stopped-flow infrared spectroscopy. (Kevin Coughlin/Brookhaven National Laboratory)
While the first two steps of this scheme are “dark reactions,” the third step that results in free methanol is initiated by the absorption of light by the ruthenium hydroxymethyl (Ru-CH2OH+) complex. The proposed mechanism by which this occurs is through an excited-state electron transfer between the Ru-CH2OH+ and a molecule of organic hydride followed rapidly by a ground proton transfer that results in the generation of methanol in solution.
“The ‘one-pot’ and selective nature of this reaction results in the generation of millimolar (mM) concentrations of methanol — the same range of concentrations as the starting materials — and avoids complications that have plagued previous efforts to use inorganic catalysts for these reactions,” said UNC co-author and CHASE Director Gerald Meyer. “This work can therefore be viewed as an important step in the use of renewable organic hydride catalysts to the decades-long quest for room temperature catalytic methanol production from CO2.”
This research was supported by the Center for Hybrid Approaches in Solar Energy to Liquid Fuels (CHASE), an Energy Innovation Hub funded by the DOE Office of Science.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
2024-21740 | INT/EXT | Newsroom