Collaboration Reveals How Light Unlocks Chemistry of Nickel Catalyst
July 1, 2025
 enlarge
enlarge
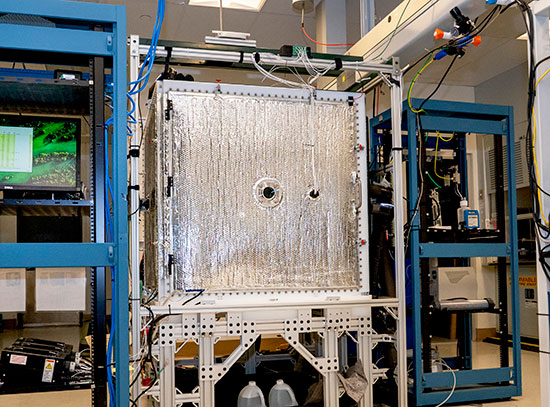
Lakshmy Kannadi Valloli of Brookhaven Lab's Chemistry Division performing experiments at Brookhaven's Laser Electron Accelerator Facility. These experiments created a solvent radical and followed the chemistry as it produced a previously unknown intermediate form of a nickel-based catalyst. (Kevin Coughlin/Brookhaven National Laboratory)
UPTON, N.Y. — A team of scientists across several U.S. Department of Energy (DOE) national laboratories has unraveled how light and a previously unknown form of certain nickel-based catalysts together unlock and preserve reactivity. This research, described in the journal Nature Communications, could potentially advance the use of abundant nickel in place of more expensive palladium in industrial chemistry.
The collaborative research effort was spearheaded by DOE’s National Renewable Energy Laboratory (NREL) and involved scientists from DOE’s SLAC National Accelerator Laboratory, Brookhaven National Laboratory, and Argonne National Laboratory, among other institutions.
 enlarge
enlarge
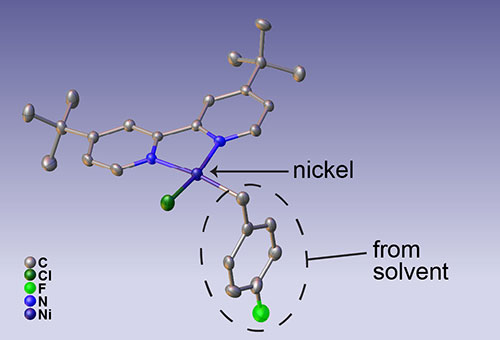
Structure of a previously unknown intermediate form of a nickel catalyst. The nickel atom is highlighted and the portion of the molecule formed when the solvent radical reacts with the activated nickel is circled. (Max Kudisch based on X-ray crystal structure determined by him and Rebecca Smaha/NREL).
Nickel catalysts have emerged as promising replacements for palladium catalysts in industrial-scale chemical reactions, as nickel is both more readily available and cheaper. Nickel has other advantages: Its reactivity can be driven by light instead of the high heat required for palladium, resulting in milder overall reaction conditions, which expands the variety of reactions that can be done. Nickel catalysts can also facilitate reactions that are new and have not been demonstrated with palladium, but key questions regarding how these light-activated nickel catalysts operate have remained unanswered until now.
The newly published paper explains how light activates the catalyst to enable it to join two fragments of simple molecules to make a more complex molecule. Along the way, the researchers discovered a new intermediate form of the nickel catalyst that keeps the catalyst from degrading.
“Pharmaceuticals is the only area that has commercialized light-driven nickel catalysis so far, but nickel-based catalysts can also potentially replace palladium catalysts for a variety of other industrial processes, including in the agricultural industry and the manufacture of electronics,” said Max Kudisch, first author of the paper and a postdoctoral researcher at NREL. “There are some very large-volume chemicals that are produced there where these sorts of methods could be applicable.”
The price difference between the two elements is vast. An ounce of nickel costs approximately 50 cents, while an ounce of palladium approaches $1,000.
“Nickel has often been used in tandem with an iridium photosensitizer,” said Matthew Bird, a chemist at Brookhaven and a co-author of the paper. “But as we start to understand exactly how it works, we could then see ways of getting rid of the iridium, a rare element like palladium, and just having the nickel. That adds to the potential value.”
 enlarge
enlarge
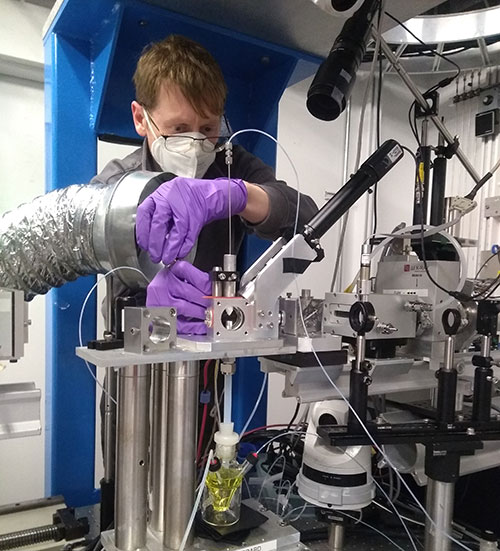
Justin D. Earley, preparing nickel/iridium solutions for time-resolved X-ray absorption measurements at the Advanced Photon Source, beamline 11ID-D, at Argonne National Laboratory. (Reid Obadiah/NREL)
The researchers experimented with nickel dihalides, compounds where nickel is bonded to two halide ions such as chloride, which are the predominant source of nickel used in these types of reactions. Exposure to light causes a bond between the nickel and chloride to break, which lowers the oxidation state of nickel and suddenly makes it reactive. But the freed chloride ion, now a chlorine “radical” due to the broken bond, does not sit idly by. In the reaction the team studied, they first hypothesized and then confirmed that it interacts with the solvent. This creates an activated form of the solvent that in turn can react with the activated nickel.
That turns out to be a crucial and previously unknown step because it forms a stable nickel intermediate that prevents the activated nickel atoms from interacting directly with one another.
“Controlling the amount of the nickel in the lower oxidation state in the reaction is essential to prevent the catalyst from getting deactivated,” Kudisch said.
If the intermediate did not exist, the lower oxidation state form of nickel would build up and bind with itself, forming a nickel compound that can no longer catalyze the reaction.
Instead, the solvent-bound intermediate can react further to complete the joining of molecules to achieve the desired chemistry.
The researchers used a range of techniques to follow the chemistry step by step, showing how light drives the chemistry.
One of these tools was the Laser Electron Accelerator Facility (LEAF) within Brookhaven Lab’s Chemistry Division, which combines very short pulses of electrons with various spectroscopic detection methods to produce and examine transient molecular and atomic species with high time resolution.
 enlarge
enlarge
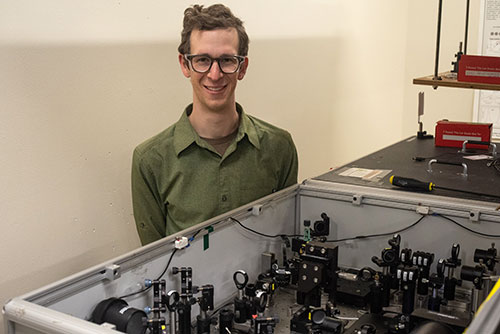
Max Kudisch in the ultrafast spectroscopy of photoconversion processes lab at DOE's National Renewable Energy Laboratory (NREL) where he performed experiments to investigate the role of light in activating the nickel pre-catalyst. (Justin Johnson/NREL)
“Pulse radiolysis lets us generate reactive intermediates to recreate a particular step in a proposed reaction mechanism to see if that step does or does not actually happen,” said Bird.
Lakshmy Kannadi Valloli, a Brookhaven Lab postdoc working with Bird, used LEAF to generate the reactive “radical” form of the solvent. “Then we watched that radical react with the nickel and saw what species it made,” Kannadi Valloli said.
The spectroscopic signature matched what Kudisch had seen when he shone light on the solution. This helped to confirm the hypothesis of how light activates the catalyst, and how the subsequent reactions generate the protective nickel intermediate.
Scientists at SLAC further characterized the intermediate using powerful X-rays at the Stanford Synchrotron Radiation Light Source (SSRL), a DOE Office of Science user facility, to understand its atomic-scale structure.
“Max made it by shining light on it. We made it by pulse radiolysis. And then our colleagues at SLAC looked at it with X-rays,” Bird said.
With those techniques all combined, we know the exact molecular structure of this intermediate form of the nickel catalyst and the pathway through which it is formed,” Kudisch concluded.
This mechanistic understanding could lead to new strategies to prevent catalyst degradation and control the amount of activated nickel catalyst present during the reaction to advance the use of light-driven nickel catalysts.
 enlarge
enlarge
The five BioLEC EFRC principal investigators on this study (left to right): Hannah Sayre (Northeastern University), Matthew Bird (Brookhaven National Laboratory), Amy Cordones (SLAC National Accelerator Laboratory),and Garry Rumbles and Obadiah Reid (both of NREL and University of Colorado Boulder).
In addition to the four national laboratories, researchers who contributed to the project are with Northeastern University and the University of Colorado, Boulder. Other NREL personnel listed as co-authors are Justin Earley, Anna Zieleniewska, Rebecca Smaha, Garry Rumbles, and Obadiah Reid.
The research was funded by DOE’s Bio-Inspired Light-Escalated Chemistry (BioLEC) Energy Frontier Research Center (EFRC) via the DOE Office of Science.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
2025-22468 | INT/EXT | Newsroom