Electrocatalyst Turns Greenhouse Gas into Pure Liquid Fuel
Rice Lab's 'green' invention reduces carbon dioxide into valuable fuels
September 30, 2019
 enlarge
enlarge
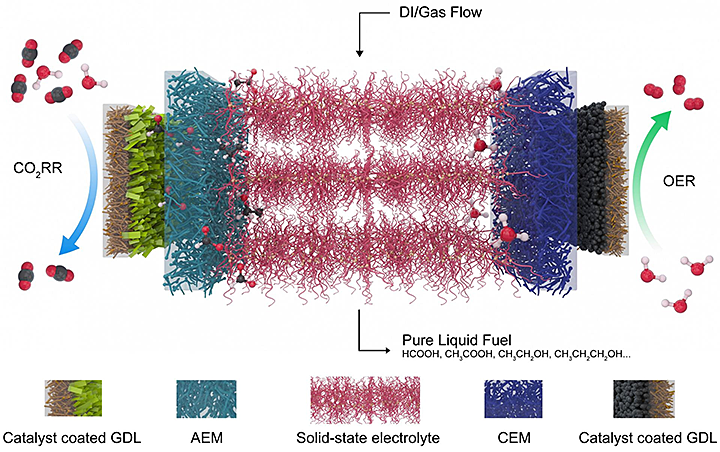
At the left is a catalyst that selects for carbon dioxide and reduces it to a negatively charged formate, which is pulled through a gas diffusion layer and the anion exchange membrane (AEM) into the central electrolyte. At the right, an oxygen evolution reaction catalyst generates positive protons from water and sends them through the cation exchange membrane. The ions recombine into formic acid or other products that are carried out of the system by deionized water and gas. (Credit: Rice University)
The Science
Scientists created and tested a new electrocatalyst that turns carbon dioxide into formic acid, which can be used directly as a liquid fuel in a fuel cell.
The Impact
This work can help repurpose a common greenhouse gas in an efficient and environmentally friendly way with an electrolyzer that uses renewable electricity to produce pure liquid fuels.
Summary
A common greenhouse gas could be repurposed in an efficient and environmentally friendly way with an electrolyzer that uses renewable electricity to produce pure liquid fuels. The catalytic reactor, developed by Rice University scientists, uses carbon dioxide as its feedstock and, in its latest prototype, produces high concentrations of highly purified formic acid. Formic acid produced by traditional carbon dioxide devices needs costly and energy-intensive purification steps. The direct production of pure formic acid solutions will help promote commercial carbon dioxide conversion technologies.
In tests, the new electrocatalyst reached an energy conversion efficiency of about 48.5 percent. Two advances made the new device possible. The first was the development of a robust 2-D bismuth catalyst and the second was a solid-state electrolyte that eliminates the need for salt as part of the reaction.
The Rice lab worked with Brookhaven National Laboratory to view the process in progress by using x-ray absorption spectroscopy, a powerful technique available at the Inner-Shell Spectroscopy (ISS) beamline at Brookhaven Lab’s National Synchrotron Light Source II (NSLS-II). The technique enables scientists to probe the electronic structure of electrocatalysts in operando—that is, during the actual chemical process.
In this work, the team followed bismuth’s oxidation states at different potentials and were able to identify the catalyst’s active state during carbon dioxide reduction.
Carbon dioxide reduction is important for its effect on global warming, as well as for green chemical synthesis. Therefore, if electricity is generated by renewable sources like the sun or wind, scientists could create a loop that turns carbon dioxide into something important, without emitting more of it, by using a technology based on these advances.
Download the research summary slide
Related Links
Feature Story: “Rice Reactor Turns Greenhouse Gas into Pure Liquid Fuel”
Contact
Haotian Wang
Rice University
htwang@rice.edu
Publications
C. Xia, P. Zhu, Q. Jiang, Y. Pan, W. Liang, E. Stavitsk, H. N. Alshareef, H. Wang, “Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices”, Nature Energy 4, 776-785 (2019). DOI: 10.1038/s41560-019-0451-x
Funding
The work was partially funded through the Center for Advanced Mathematics for Energy Research Applications (CAMERA), which is jointly funded by the Advanced Scientific Computing Research (ASCR) and Basic Energy Sciences (BES) within the Department of Energy’s Office of Science, under Contract No. DE-AC02-05CH11231. This work was conducted at Lawrence Berkeley National Laboratory and Brookhaven National Laboratory. This research used resources of the Center for Functional Nanomaterials and the National Synchrotron Light Source II, which are U.S. DOE Office of Science Facilities, at Brookhaven National Laboratory under Contract No. DE-SC0012704.
2019-16847 | INT/EXT | Newsroom