Copper Nanosheets as Improved Electrochemical Catalysts
Scientists synthesized free-standing nanosheets that reduce carbon monoxide and dioxide to usable multicarbon products
May 31, 2019
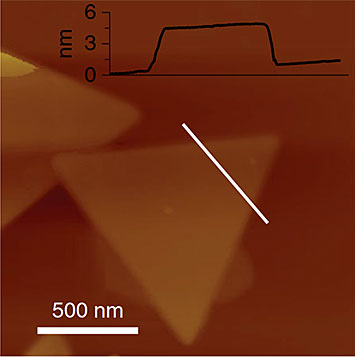
Atomic force microscope image showing the thickness of a single Cu nanosheet. Image credit: Nat. Catal. 2, 423–430 (2019)
The Science
Freestanding high-quality copper (Cu) nanosheets were synthesized for the electrochemical reduction of carbon monoxide (CO) and carbon dioxide (CO2) to acetate.
The Impact
CO2 electrolysis is attractive for sustainable fuel and chemical production. These findings offer new insights into rational design of CO2/CO reduction catalysts.
Summary
Converting carbon dioxide (CO2) and carbon monoxide (CO) to high-value multicarbon (C2+) products through electrochemical reduction is one promising avenue for fuel and chemical production while reducing the amount of CO2 in our atmosphere.
Among all the monometallic catalysts, copper (Cu) has attracted much attention because of its unique ability to convert CO2 or CO into C2+ products with an appreciable selectivity. However, so far, the synthesis of Cu materials that expose the desired facets, the 111 surface, for high selectivity remained a challenge.
In this study, scientists report a facile synthesis of freestanding triangular-shaped two-dimensional Cu nanosheets that selectively expose the (111) surface. After the successful synthesis of the nanosheets, the scientists investigated their catalytic properties.
In electrolyte, the synthesized Cu nanosheets exhibited an acetate Faradaic efficiency of 48% with an acetate partial current density up to 131 mA cm−2 in electrochemical CO reduction. The scientists further concluded that the high acetate selectivity, which is preferred for C2+ chemical production, is attributed to the suppression of ethylene and ethanol formation, probably due to the reduction of exposed (100) and (110) surfaces.
The scientists used operando x-ray absorption spectroscopy at the Inner-Shell Spectroscopy (ISS) beamline of the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory, to reveal the structural stability and the chemical state of the nanosheets during catalysis. The findings of this study offer a new path to improved, cost-effective electrolyzer designs, devices that use an electric current to provide the energy that splits a water molecule (H2O) into hydrogen (H2) and oxygen (O2).
Download the research summary slide
Contact
Feng Jiao
University of Delaware
jiao@udel.edu
Yijin Kangy
University of Electronic Science and Technology of China
kangyijin@uestc.edu.cn
Publications
W. Luc, X. Fu, J. Shi, J.-J. Lv, M. Jouny, B. H. Ko, Y. Xu, Q. Tu, X. Hu, J Wu, Q. Yue, Y. Liu, F. Jiao, Y. Kang, “Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate”, Nat. Catal. 2, 423–430 (2019). DOI: 10.1038/s41929-019-0269-8
Funding
This work is supported by the Department of Energy (USA) under Award no. DEFE0029868 and the National Natural Science Foundation of China under Award nos 51601030 and 21773023, by Science Foundation Faculty Early Career Development program (Award no. CBET-1350911). This work is supported by the International Institute for Nanotechnology (IIN) and Institute for Sustainability and Energy (ISEN) at Northwestern University. The theoretical calculation is supported by the Welch Foundation (Grant no. F-1959-20180324) and the startup grant from UT Austin, and used computational resources sponsored by the DOE’s Office of Energy Efficiency and Renewable Energy and located at the National Renewable Energy Laboratory, and the Texas Advanced Computing Center (TACC) at UT Austin. This work made use of the Electron Probe Instrumentation Center (EPIC) facility of Northwestern University’s Atomic and Nanoscale Characterization Experimental Center (NUANCE), which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the Materials Research Science and Engineering Centers (MRSEC) program (NSF DMR-1121262) at the Materials Research Center; the IIN. This work made use of the J.B. Cohen X-Ray Diffraction Facility supported by MRSEC and SHyNE. This research used resources at the Inner-Shell Spectroscopy (ISS) beamline 8-ID of the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract no. DE-SC0012704.
2019-17169 | INT/EXT | Newsroom









