Researchers "Watch" Molten Salts Carve Tiny Nooks and Tunnels into Metal Alloys in 3D
The study helps explain corrosion by molten salts, knowledge that is critical for developments in nuclear and solar energy technologies
September 22, 2021
 enlarge
enlarge
The research team from Brookhaven National Laboratory (BNL), Stony Brook University (SBU) and the National Institute of Standards and Technology (NIST): Karen Chen-Wiegart (SBU/BNL), Bruce Ravel (NIST), James F. Wishart (BNL), Lin-Chieh Yu (SBU), Arthur Ronne (SBU), Xiaoyang Liu (SBU), Yang Liu (SBU), Cheng-Hung Lin (SBU), Wah-Keat Lee (BNL), Xianghui Xiao (BNL), Mingyuan Ge (BNL), Stephen Antonelli (BNL). Bobby Layne (BNL) and Anatoly I. Frenkel (SBU/BNL) also worked on the study (not pictured).
A multidisciplinary team of scientists has used the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User facility located at the DOE’s Brookhaven National Laboratory, to investigate how high-temperature molten salts corrode metal alloys. The group found a novel approach for using molten salts to create porous metallic materials with microscopic networks of voids and metal ligaments, which could have applications in a variety of fields, such as energy storage and sensing. Their work also supports the development of molten salt reactors (MSRs), a technology that could produce safer, cheaper, and more environmentally sustainable nuclear power.
Molten salts are one of the leading candidates as a medium for high-temperature heat transfer in a variety of applications, including next-generation nuclear and concentrated solar power plants. They have several features that make them desirable, such as high boiling points, high specific heats, high thermal conductivities, and low vapor pressures. However, one of the challenges of molten salts is their corrosivity when they come in contact with alloys.
In MSRs, the molten salt contains the nuclear fuel in dissolved form and also serves as the primary heat transfer fluid, operating at 500 – 900°C (about 930 – 1650°F). One of the key steps toward developing MSRs is to gain a firm understanding of the chemistry of molten salts and how they interact with the structural materials in a reactor at high temperatures, with their corrosive effects being a main focus. This work helps to address that goal by providing insight into molten salt dealloying, a process by which certain elements within a metal alloy are preferentially leached away into the molten salt during corrosion. It is the first study that explores using the corrosive nature of molten salts to dealloy and purposely create porous structures.
 enlarge
enlarge
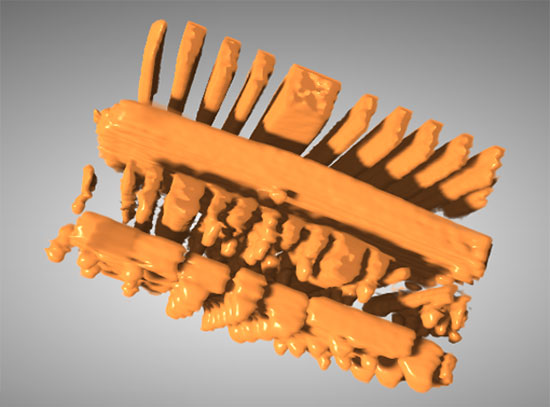
The illustration shows how molten salt over time removes metallic material (blue) from the sample in 3D (upper) and 2D visualizations (lower). This process, so-called 'dealloying' creates a bicontinuous porous metallic material with networks of voids (orange). Starting from left to right the image shows the removal of more material with passing reaction time.
The research, which is described in a paper published on June 9, 2021 in Nature Communications, results from a collaboration between NSLS-II and the Brookhaven-led Molten Salts in Extreme Environments Energy Frontier Research Center (MSEE EFRC). EFRCs were established by DOE’s Office of Basic Energy Sciences to bring large teams together to tackle complicated and interdisciplinary fundamental research challenges for the advancement of energy technologies. The MSEE team on this work included members from Stony Brook University, Brookhaven’s Chemistry Division, and Oak Ridge National Laboratory.
“The mission of MSEE is to provide the fundamental molten salt science needed to enable MSR technology,” said the director of MSEE and one of the paper’s authors, Brookhaven chemist James Wishart.
The work was done at two NSLS-II beamlines, the Full-Field X-Ray Imaging (FXI) beamline and the Beamline for Materials Measurement (BMM).
“The FXI beamline features an imaging technique called 3D x-ray nanotomography, which yields a time series of 3D visualizations — essentially a 3D movie — of a sample’s internal structure with a resolution of tens of nanometers,” said the lead scientist at the FXI beamline, Wah-Keat Lee, who is also an author. “Other facilities have similar instruments, but FXI can yield images 20 times faster. This is what makes this beamline so useful for studies like this one.”
 enlarge
enlarge
(From left to right) The research team from Oak Ridge National Laboratory (ORNL) and University of Tennessee (TU): Sheng Dai (ORNL/UT), Alexander S. Ivanov (ORNL), Phillip Halstenberg (ORNL/UT), Shannon M. Mahurin (ORNL), Dmitry S. Maltsev (UT).
Both FXI and BMM provide another technique called x-ray absorption near-edge structure (XANES) spectroscopy, which is used to yield information on the oxidation state and local structure of the alloy elements during the dealloying reaction. The experimental results were then complemented by computational modeling and simulation.
To be able to image high-temperature molten salt corrosion, the FXI beamline staff, NSLS-II engineers, and the MSEE research team jointly developed a special miniature heater that enables real-time measurements while materials are evolving at conditions up to 1000 °C. This was a major accomplishment on its own that was documented in a recent paper, published in Journal of Synchrotron Radiation.
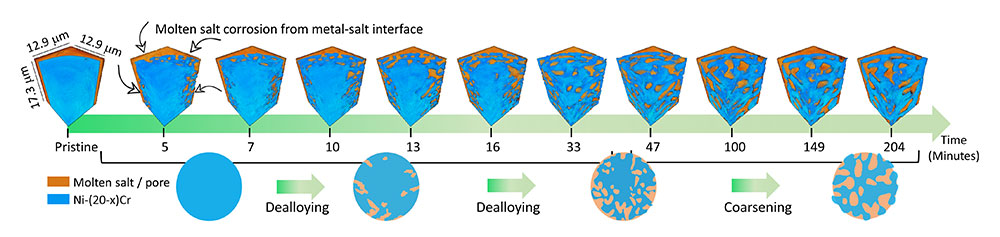
The team used the FXI heater system to time-resolve the morphological evolution of a nickel-chromium alloy (80% Ni / 20% Cr) wire in a molten 50-50 mixture of potassium chloride and magnesium chloride at 800 °C. As time progressed, chromium was leached out of the wire by corrosion and the remaining nickel restructured into a porous network. This is the first time that researchers have observed the changing 3D structure of a material undergoing the dealloying process as it is happening.
“We watched the sample change in front of our eyes and were able to take a video of every single step, which is remarkable,” said Stony Brook PhD candidate Xiaoyang Liu, one of the joint first authors of the paper.
The team observed that the dealloying process first begins at the interface between the alloy and the salt and propagates through to the center of the alloy, creating the pore network. As chromium is further leached away into the molten salt, the pores and cavities become larger (which is called “coarsening”) as a result of the diffusion of Ni atoms on the surface of the alloy.
The three-dimensional morphology of the material formed in this study is classified as “bicontinuous,” meaning both phases — the alloy and the network of pores created by the salt corrosion — are continuous and unbroken. Porous bicontinuous materials are of great interest to researchers due to their reduced weight, large surface areas, ability for mass transport of fluids through the pores, and electrical or thermal conductivity through the material matrix. Bicontinuous metal alloys, especially ones with fine pore sizes, have numerous potential applications in several fields, including energy storage, sensing, and catalysis.
The video shows the change in the metallic material (blue) that is being dealloyed using molten salt at different times during the process. In each step we can see how more voids (orange) are being created within the material, forming a continuous network.
Several methods have historically been employed to create these highly sought-after materials, including acid etching of the most easily corroded element, or selective dissolution in liquid metal. However, the molten salt approach, which has not been previously explored, operates by different mechanisms and follows different rules that can provide a higher degree of control of both the leaching and restructuring processes, potentially resulting in superior materials. This degree of control is possible because the imaging capabilities at the FXI beamline allow the researchers to quantify the rates of the dealloying and coarsening processes as they change parameters such as temperature and alloy and salt composition.
“The FXI beamline was absolutely critical to this work,” said Stony Brook PhD student Arthur Ronne, the other joint first author and co-corresponding author. “Its time resolution, with the ability to watch the structure change on the minute scale at an excellent nanoscale spatial resolution, along with the furnace we jointly built, made this study possible.”
This work, and its continuing extension into the effects of temperature and salt and alloy composition, is very important for the design of durable molten salt reactor systems, which span a range of temperatures where mechanisms of corrosion by these processes could be predicted to vary in different locations, and also depend on the contents of the fuel salt. The team will use the FXI beamline and other advanced techniques to obtain the necessary mechanistic information to enable such predictions. In doing so, they will obtain key information to guide the deliberate preparation of bicontinuous alloy materials with specific morphologies and properties for a wide range of applications.
“Behind this work is a multitude of incredible scientists and engineers,” said corresponding author Karen Chen-Wiegart, an assistant professor in Stony Brook's College of Engineering and Applied Sciences who holds a joint appointment at NSLS-II. “It was only through the partnership of a large research center like MSEE and a world-class facility like NSLS-II that we were able to take this step. We are really only at the beginning of a wonderful journey to further explore the complex and yet fascinating interactions between the materials and molten salts using advanced synchrotron techniques!”
Brookhaven National Laboratory is supported by the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.
Follow @BrookhavenLab on Twitter or find us on Facebook.
2021-19113 | INT/EXT | Newsroom