Probing Active-Site Chemical States in a Co-Based Catalyst
March 4, 2024
 enlarge
enlarge
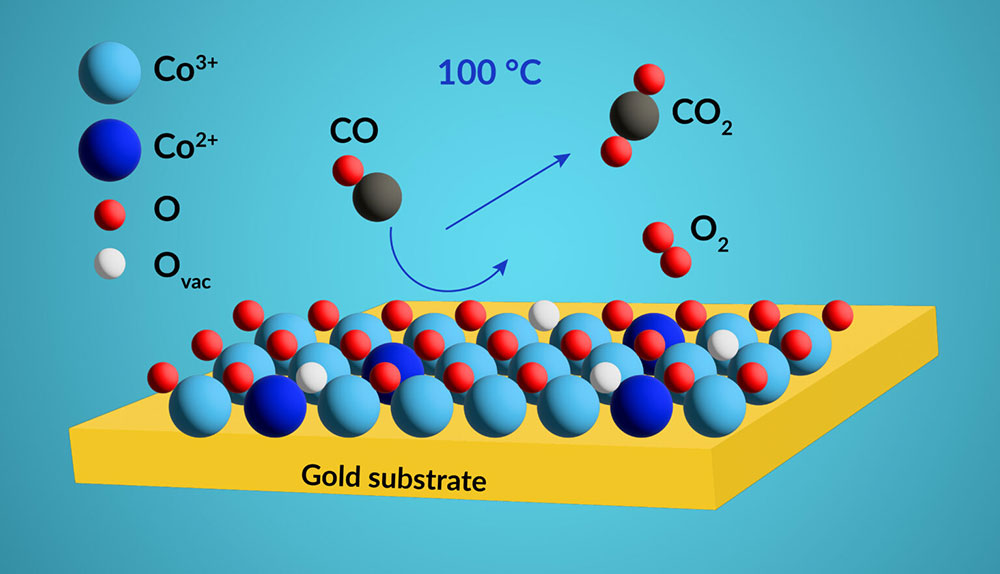
Carbon monoxide (CO) is converted to carbon dioxide (CO2) by a thin layer of cobalt oxide on a gold substrate. Under oxygen-rich conditions, a phase containing trivalent cobalt (Co3+) is formed that catalyzes the reaction effectively over a wide range of temperatures.
The following science highlight was originally issued by the Advanced Light Source (ALS), a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Lawrence Berkeley National Laboratory. Some of the ambient-pressure x-ray photoelectron spectroscopy studies, which probed the chemical-state changes in CoOx under reaction conditions, were performed at the In situ and Operando Soft X-ray Spectroscopy (IOS) beamline at the National Synchrotron Light Source II (NSLS-II), a DOE Office of Science User Facility at DOE’s Brookhaven National Laboratory. For more information on Brookhaven’s role in this research, contact Denise Yazak (dyazak@bnl.gov, 631-344-6371).
Scientific Achievement
Researchers identified the dominant chemical state of active sites in a cobalt-based catalyst using resonant photoemission spectroscopy under realistic conditions at the Advanced Light Source (ALS).
Significance And Impact
The work will help scientists develop more-efficient catalysts for removing noxious carbon monoxide gas from exhaust streams generated by the burning of fossil fuels.
Carbon monoxide (CO) is converted to carbon dioxide (CO2) by a thin layer of cobalt oxide on a gold substrate. Under oxygen-rich conditions, a phase containing trivalent cobalt (Co3+) is formed that catalyzes the reaction effectively over a wide range of temperatures.
Quest for maximum efficiency
Catalytic converters have been successfully used for decades to remove noxious gases from exhaust streams produced by fossil-fuel combustion. The central chemical reaction, the conversion of carbon monoxide (CO) to carbon dioxide (CO2), requires a catalyst that provides special sites where the reactants and products can bind, react, and detach without requiring much energy. In particular, noble metals such as gold or platinum, when combined with “reducible” oxides (i.e., oxygen-containing materials that can exchange electrons with the metal), have been found to exhibit superior catalytic activity. However, to maximize efficiency, scientists need a clearer understanding of the origins of this increased activity.
To this end, researchers used resonant photoemission spectroscopy (ResPES) at the ALS to study thin films of cobalt oxide (CoOx) on a gold substrate. The findings provide new insight into the enhanced catalytic reactivity of CoOx catalysts, highlighting both their chemical and topographical evolution in response to varied ambient-pressure environments.
Topographic and chemical restructuring
In previous work done at the ALS, the researchers revealed that many catalysts (platinum, copper, and gold/silver alloys) rearrange their surfaces in response to ambient conditions. More recently, the group explored gains in efficiency when pairing noble metals with transition-metal oxides, particularly CoOx. When gold and CoOx come into contact with one another, they exchange electrons, which means that the Co can have several oxidation states (e.g., Co0, Co2+, and Co3+). How does this “chemical restructuring” affect catalytic efficiency and under what conditions (temperatures, gas composition) is efficiency maximized?
Answering these questions requires information about Co oxidation states. However, while ambient-pressure x-ray photoelectron spectroscopy (APXPS) can directly probe chemical-state changes in CoOx under reaction conditions, the Co2+ and Co3+ spectroscopic peaks are extremely close together and difficult to distinguish. Therefore, the researchers turned to another ambient-pressure technique, ResPES, which uses resonant photoelectron emission to enhance spectroscopic signals. The ResPES experiments were conducted at ALS Beamline 9.3.2. APXPS studies were also performed at the ALS, NSLS-II, and SSRL. Density functional theory calculations provided deeper insights into reaction pathways and free-energy barriers for the reactions.
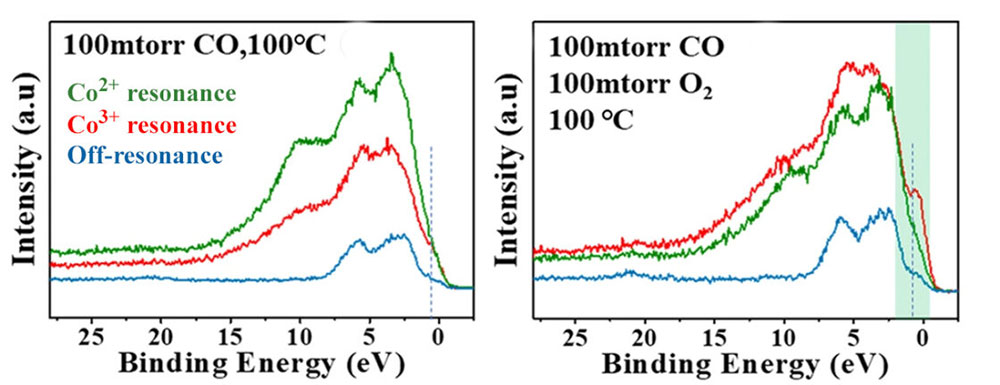
Resonant valence-band photoelectron spectra of CoOx film on a substrate of Au(111) under CO exposure (left) and under CO oxidation conditions (right). Green and red spectra correspond to Co2+ and Co3+ resonant excitation energies, while the blue spectrum was measured off-resonance. With resonant enhancement, it can be clearly distinguished that the Co3+ sites contribute the most to catalytic activity under CO oxidation conditions.
 enlarge
enlarge
Resonant valence-band photoelectron spectra of CoOx film on a substrate of Au(111) under CO exposure (left) and under CO oxidation conditions (right). Green and red spectra correspond to Co2+ and Co3+ resonant excitation energies, while the blue spectrum was measured off-resonance. With resonant enhancement, it can be clearly distinguished that the Co3+ sites contribute the most to catalytic activity under CO oxidation conditions.
Trivalent cobalt dominates
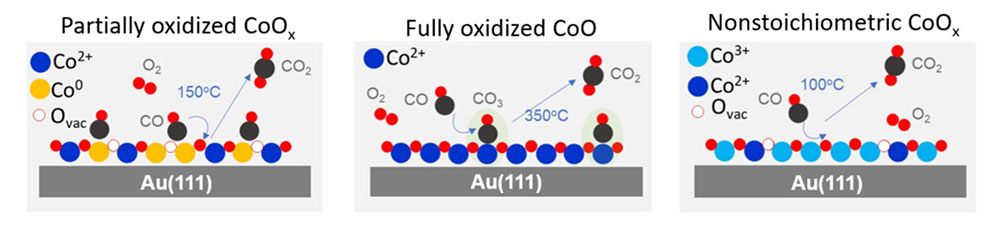
The results revealed that three reaction regimes exist in response to chemical and topographic restructuring of the CoOx: partially oxidized CoOx (x<1), fully oxidized CoO, and nonstoichiometric CoOx (x>1). Under oxygen-lean conditions and moderate temperatures (≤150 °C), the partially oxidized films were found to be efficient catalysts. Fully oxidized CoO films (containing only Co2+) form carbonates in the presence of CO that poison the reaction below 300 °C. Under oxygen-rich conditions, a more oxidized, nonstoichiometric CoOx phase (x>1) containing both Co2+ and Co3+ appears. For this case, the ResPES data demonstrated that Co3+ is the most active catalyst site over a wide temperature range.
Identifying the nature of the active site and how to “turn it on” is key to catalyst design, and these findings provide scientists with new avenues for the design of more-efficient catalysts. Future plans include repeating the experiment using a platinum substrate instead of gold and comparing the results. The ultimate goal is to eventually unravel the mystery of what drives the strong interaction between metal and support materials in catalysts.
Schematic illustration of proposed CO oxidation reaction pathways on CoOx films on Au(111) in three structurally different phases.
 enlarge
enlarge
Schematic illustration of proposed CO oxidation reaction pathways on CoOx films on Au(111) in three structurally different phases.
Contacts: Miquel Salmeron and Hao Chen
Researchers: H. Chen, L.J. Falling, J. Oliver-Meseguer, M. Jaugstetter (Berkeley Lab); H. Kersell (ALS and Oregon State University); G. Yan and P. Sautet (UCLA); X. Zhao, A.T. Bell, and M. Salmeron (Berkeley Lab and UC Berkeley); S. Nemsak (ALS and UC Davis); A. Hunt and I. Waluyo (NSLS-II); and H. Ogasawara (SLAC).
Funding: US Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES); Alexander von Humboldt Foundation; National Science Foundation; and US-Israel Binational Science Foundation. Operation of the ALS, NSLS-II, and SSRL is supported by DOE BES.
Publication: H. Chen, L.J. Falling, H. Kersell, G. Yan, X. Zhao, J. Oliver-Meseguer, M. Jaugstetter, S. Nemsak, A. Hunt, I. Waluyo, H. Ogasawara, A.T. Bell, P. Sautet, and M. Salmeron, “Elucidating the active phases of CoOx films on Au(111) in the CO oxidation reaction,” Nat. Commun. 14, 6889 (2023), doi:10.1038/s41467-023-42301-7.
2024-21760 | INT/EXT | Newsroom