Studying the Effects of 'Microstrain' on Battery Materials
February 12, 2025
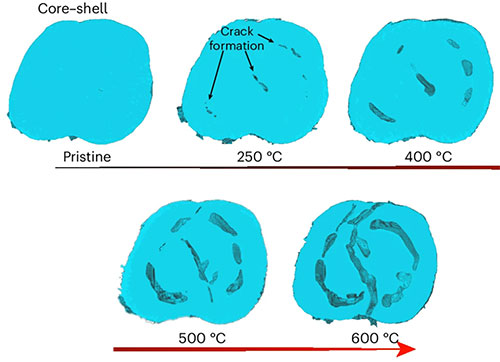 enlarge
enlarge
In situ 3D TXM-XANES results of the particles during calcination, tracking real-time bulk structural changes.
The Science
Researchers uncover insight into how structural defects form and affect the performance of two battery cathode materials.
The Impact
Understanding the "microstrain" caused by these defects could lead to ways to craft more stable battery materials.
Summary
One of the roadblocks to more energy-dense, longer-lasting battery materials are the structural defects that tend to occur during the synthesis of those materials. These defects produce an overall instability known as microstrain, which has been identified as playing a critical role in the degradation of layered transition metal oxides, a class of cathode materials used in alkali-ion batteries, such as sodium-ion and lithium-ion.
To be able to design and create battery materials that are defect-free, researchers need to understand much more about the origin and effects of microstrain. As a step toward this goal, researchers from the U.S. Department of Energy's (DOE) Brookhaven National Laboratory and Argonne National Laboratory have studied a battery cathode material during synthesis, watching the process in real time. To do this, they used X-ray and microscopy techniques at Argonne's Advanced Photon Source (APS) and Brookhaven's National Synchrotron Light Source II (NSLS-II). Both APS and NSLS-II are DOE Office of Science user facilities.
The materials studied here are two types of a sodium layered oxide, which is grown via the use of a precursor material, a layered transition metal hydroxide. As part of the growth process, secondary particles form and then undergo calcination. At NSLS-II's Full Field X-ray Imaging (FXI) beamline, the researchers studied the particles using a full-field 3D transmission X-ray microscope (TXM) in X-ray absorption near-edge structure (XANES) mode, a technique that combines two X-ray approaches to yield 3D information on the chemical states of a sample.
TXM-XANES allowed the group to see structural and chemical changes that occurred under realistic synthesis temperatures and other conditions. From the data they collected at both facilities, the group learned that as the material "grows," the spatial distribution of transition metals within the particles strongly guides several aspects of the synthesis process, including phase transformation at the nanoscale, local charge distribution, and the accumulation of microstrain. This dominance of the transition metals was another result that the researchers did not expect to see. Together, these insights are a critical step toward a better synthesis route for sodium layered oxides that could result in materials with reduced microstrain and defects, and thus significantly improved structural stability.
Related Links
Microstrain screening towards defect-less layered transition metal oxide cathodes
Contact
Xianghui Xiao
NSLS-II, Brookhaven National Laboratory
xiao@bnl.gov
Khalil Amine
Argonne National Laboratory
amine@anl.gov
Gui-Liang Xu
Argonne National Laboratory
xug@anl.gov
Publications
Wenhua Zuo, Jihyeon Gim, Tianyi Li, Dewen Hou, Yibo Gao, Shiyuan Zhou, Chen Zhao, Xin Jia, Zhenzhen Yang, Yuzi Liu, Wenqian Xu, Xianghui Xiao, Gui-Liang Xu & Khalil Amine. "Microstrain screening towards defect-less layered transition metal oxide cathodes." Nature Nanotechnology 19, pages 1644–1653 (2024) DOI: https://doi.org/10.1038/s41565-024-01734-x
Funding
Research at Argonne National Laboratory was funded by the US Department of Energy (DOE), Vehicle Technologies Office. Support from T. Duong of the US DOE’s Office of Vehicle Technologies Program is gratefully acknowledged. Use of the Advanced Photon Source and the Center for Nanoscale Materials, both Office of Science user facilities at Argonne National Laboratory, was supported by the US DOE, Office of Science and Office of Basic Energy Sciences, under contract number DE-AC02-06CH11357. This research used resources of the Argonne Leadership Computing Facility, a US DOE Office of Science user facility at Argonne National Laboratory and is based on research supported by the US DOE Office of Science–Advanced Scientific Computing Research Program, under contract number DE-AC02-06CH11357. This research used beamline 18-ID of the National Synchrotron Light Source II, a US DOE Office of Science user facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract number DE-SC0012704.
2025-22364 | INT/EXT | Newsroom









