Trace Amounts of Nickel Boost Silver's Selectivity for Essential Catalysis
June 18, 2025
 enlarge
enlarge
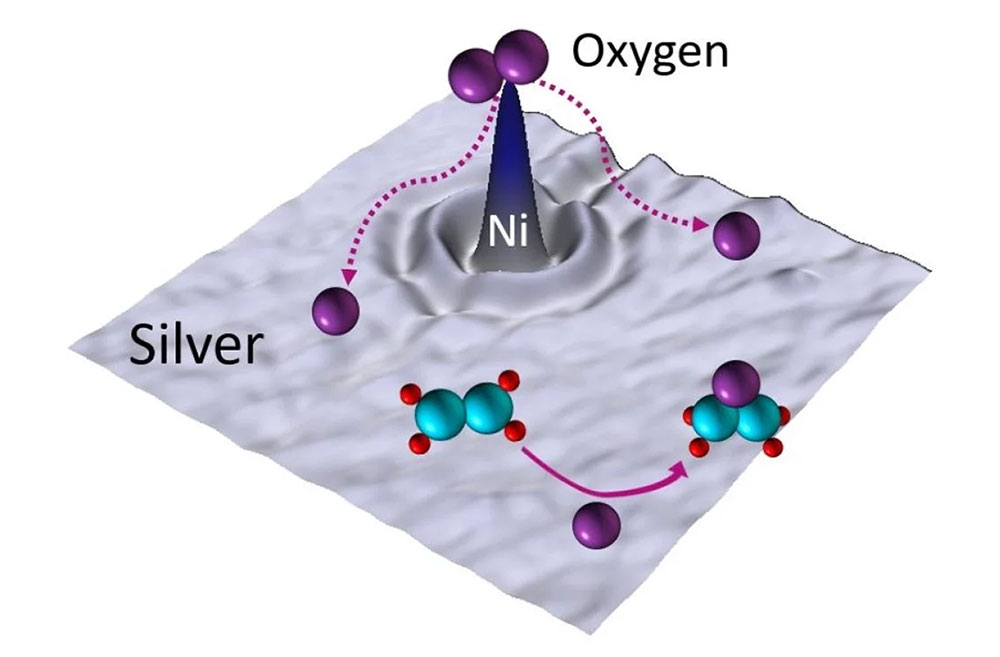
Nickel atoms on the surface of the silver particle enhance oxygen dissociation, enabling the efficient production of ethylene oxide.
The Science
A theory-based search revealed that trace amounts of nickel (Ni) boost the selectivity of ethylene epoxidation on silver (Ag), a chemical reaction that turns ethylene into a useful compound called ethylene oxide (EO), about 25% more efficiently.
The Impact
Ethylene oxide, used in products like plastics and antifreeze, has a $40B global market. Improving reaction selectivity by just 1% could save ~$200M annually by reducing ethylene loss to combustion.
Summary
A research team led by Tufts University has developed a more efficient and cost-effective method for producing ethylene oxide, a widely used industrial chemical found in plastics, antifreeze, textiles, and disinfectants. The conventional manufacturing process relies on chlorine, a naturally corrosive and expensive chemical that also generates harmful byproducts.
Using their "single-atom alloy" strategy, the team’s initial theoretical work predicted that adding trace amounts of nickel to a standard silver catalyst should significantly improve oxygen activation and spillover in ethylene epoxidation, a reaction that converts ethylene into ethylene oxide. Nickel, a low-cost and abundant metal, had not previously been explored in this context, likely due to difficulties in incorporating it into the catalyst structure.
Subsequent surface science studies and atomic-scale imaging confirmed the formation of a silver-nickel single-atom alloy. These experiments also demonstrated that the reaction could proceed more efficiently. X-ray photoelectron spectroscopy revealed that nickel helps stabilize nucleophilic oxygen, through to be responsible for unselective oxidation, thereby reducing total combustion without hindering epoxidation.
This chlorine-free approach improves reaction selectivity by 25% and could have significant economic impact. In the $40 billion global market for ethylene oxide, even a 1% increase in reaction selectivity could translate to savings of approximately $200 million annually by minimizing ethylene lost to combustion. A U.S. provisional patent has currently been filed.
Download the research summary slide (PDF)
Related Links
- Nickel promotes selective ethylene epoxidation on silver (Paper)
- Researchers discover potentially cleaner way to make an important chemical
Contact
Matthew Montemore
Tulane University
mmontemore@tulane.edu
Phillip Christopher
UC Santa Barbara
pchristopher@ucsb.edu
Charles Sykes
Tufts University
charles.sykes@tufts.edu
Publications
Jalil, E. Happel, L. Cramer, A. Hunt, A.S. Hoffman, I. Waluyo, M.M. Montemore, P. Christopher, E.C.H. Sykes, Nickel promotes selective ethylene epoxidation on silver. Science 387,869-873(2025). DOI:10.1126/science.adt1213
Funding
A.J. and P.C. acknowledge primary financial support from the US Department of Energy, BES, Catalysis Science program under contract DE-SC0021124. E.E.H., L.C., and E.C.H.S. acknowledge primary financial support from the US Department of Energy, BES, CPIMS program under contract DE-SC0004738. M.M.M. acknowledges support from Tulane University and the National Science Foundation through grant CHE-2154952. A.S.H., part of Co-ACCESS and part of the SUNCAT Center for Interface Science and Catalysis, is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division. The use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract DE-AC02-76SF00515. Use of the ICP and microscopy equipment in the UCSB MRL Shared Experimental Facilities is acknowledged, which are supported by the MRSEC Program of the National Science Foundation under award DMR 1720256. This research used resources of the 23-ID-2 (IOS) beamline of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. High performance computing resources and services were provided by Technology Services at Tulane University, New Orleans, LA, USA.
2025-22526 | INT/EXT | Newsroom









