The Surprising Ooze Factor of Glass
April 30, 2013
Reach for a tall glass of iced tea. Don't drink. Look at the glass instead.
The glass is an amorphous solid, consisting of molecules jumbled in disarray. It's the complete opposite of the ice in your drink. Ice is a crystalline solid made up of water molecules arranged in a repeat pattern.
In the world of science, glasses are solids that have a non-crystalline structure.
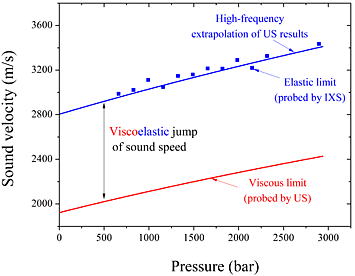
The viscoelasticity of glycerol can be seen by comparing the low-frequency limit of sound speed measured at different pressures by ultrasound (US) techniques (dashed line), the high-frequency limit measured by IXS (solid squares), and the high-frequency extrapolation of US results (solid line).
"The structure of a glass is frozen," said Alessandro Cunsolo, physicist in the Photon Sciences Directorate. "You can also think of glass as a type of fluid in which viscosity is so high that orderly arrangements of molecules are stopped."
Another property of glass is that it undergoes a so-called glass-liquid transition. Cunsolo explained that the transition from liquid to solid in a material that forms glass is exceptional – unlike water freezing as crystalline ice, a glass former remains amorphous as it cools to solid. That physical change of a liquid making a transition to an amorphous solid has puzzled scientists for a long time.
"You would not expect any global rearrangement of the molecules in a glass. But still, you observe a relaxation process," said Cunsolo. "This means that if you perturb the glass equilibrium, for instance, with a sound wave, the latter decays within a finite time. Something mysterious is happening since the decay has seemingly nothing to do with molecular movement, as would be the case with most liquids. Instead, it is connected to a static property: the lack of order in the structure."
Cunsolo uses spectroscopy to study fluids. He has published several papers on glass-forming systems, more recently a publication he co-authored with Bogdan Leu, Ayman Said and Yong Cai in the Journal of Chemical Physics (2011), and a solo paper Cunsolo published in the Journal of Physics: Condensed Matter (2012). That latest paper describes the results of inelastic X-ray scattering (IXS) measurements on a sample of glycerol at various pressures. Glycerol is a glass-forming liquid.
Cunsolo determined the ability of glycerol to support the propagation of high-frequency acoustic waves by comparing IXS spectra measured at different pressures with ultrasound absorption data. He was able to infer the presence of two distinct relaxation processes: a slow one and a fast one. The slow relaxation triggers an increase of the frequency-dependent sound velocity. The fast relaxation induces no visible dispersive effects on sound propagation. According to Cunsolo, these apparently opposite behaviors are likely to coexist in all glass formers near the temperatures at which they melt into liquids.
"This is a novel result because it provides a quantitative understanding of the measured sound dispersion using recently developed theory," said Cunsolo. "Thus, it potentially solves a long-standing debate about the dynamics of glass formers."
Cunsolo joined the Photon Sciences Directorate in 2009 to help develop the Inelastic X-ray Scattering (IXS) beamline at the National Synchrotron Light Source II (NSLS-II). He has done his research using the Advanced Photon Source at Argonne National Laboratory. When NSLS-II comes on line in 2015 for early science experiments, Cunsolo is looking forward to using the IXS beamline's new high-resolution and high-contrast spectrometer to continue his work.
"One of the most controversial issues revolves around the hypothesis that high-frequency excitations remain trapped inside local regions of the sample, instead of propagating throughout it," said Cunsolo. "Improved resolution will provide a more accurate measurement of the spectral shape, which will help settle many open issues on high-frequency excitations in glass formers."
2013-3892 | INT/EXT | Newsroom









