NSLS Enables Critical Assessment of Proposed Solar Material
September 11, 2012
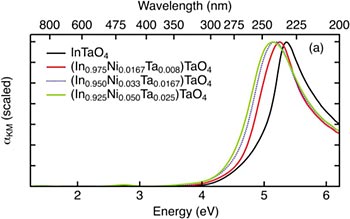
Optical absorption data of InTaO4 for different levels of doping. The curves show that Ni doping does not significantly lower the band gap, which remains at nearly 4 eV. Most solar energy is found at 3 eV and below.
In a study conducted in part at NSLS, a research group has gained valuable information about a material being investigated for use in an emerging technology for renewable energy production: using sunlight-absorbing semiconductors to split water molecules and yield hydrogen gas, which can be fed into a fuel cell to generate electricity or used as fuel itself. The group focused on a semiconductor that was deemed to be promising, but concluded that it has a composition different from what was previously suggested and is therefore not a good absorber of sunlight.
As a result, the biggest roadblock faced by this technology is the still the lack of a suitable semiconductor. There are few candidates and, to date, the best of them convert sunlight into hydrogen at a rate of less than one percent, which is far too low for real-life applications.
The material studied here is indium tantalate (InTaO4) – a compound of the elements indium, tantalum, and oxygen – that was “doped” with nickel (Ni) atoms. The group includes scientists from Stony Brook University, Brookhaven National Laboratory, Oak Ridge National Laboratory, and the National Institute of Standards and Technology.
At beamlines X16C and X19A, the researchers studied how the Ni atoms are incorporated into InTaO4 and also investigated its magnetic properties. One technique they used is x-ray absorption near-edge spectroscopy (XANES), which revealed that the Ni atoms take the form of a positive ion, Ni2+, rather than the Ni3+ that was previously suggested. Each Ni2+ co-substitutes with Ta5+ for an In atom (which is actually an In3+ ion), rather than through the direct replacement of In3+ that was previously hypothesized.
The group also employed x-ray diffraction to determine the atomic composition of the material for different amounts of Ni. They also used neutron diffraction at Oak Ridge’s Spallation Neutron Source to confirm this mechanism.
Ultimately, the group found that Ni-doped InTaO4 does not have the intrinsic properties it needs to absorb visible light, even at the maximum doping concentration of 12 percent. This conclusion is a result of the material’s band gap, the threshold minimum energy (measured in electron volts, or eV) for the semiconductor to absorb and harness light for energy production. Semiconductors with band gaps above 3.0 eV cannot effectively absorb sunlight. The group measured a band gap no lower than 3.7 eV for Ni-substituted InTaO4, refuting the findings of an earlier study that reported a band gap of 2.3 eV. Those researchers may have incorrectly attributed the material’s light-absorbing ability to an impurity in the system that was not identified due to the prior lack of knowledge about the true Ni substitution mechanism in this system.
Converting sunlight into hydrogen rather than electricity can have substantial advantages. The electrical output of conventional solar cells can vary greatly with weather and seasons, and tying them into the national electric grid – thus making them useful on a large scale – is not yet an easy task due to the challenges of storing large amounts of electrical energy. Making a storable and transportable chemical fuel such as hydrogen may be a smarter approach, yielding, as the authors say, a more “versatile energy currency.”
This study is published in the April 24, 2012, online edition of Inorganic Chemistry.
2012-3345 | INT/EXT | Newsroom









