A Nanomaterial in 3-D
June 6, 2005
This is an informational posting, not a Brookhaven press release.
Upton, NY - Scientists from the U.S. Department of Energy's Brookhaven National Laboratory, Central Michigan University, and Michigan State University have determined the three-dimensional molecular structure of a material that is very promising for a variety of real-world applications, including more efficient solar-energy cells and biosensors, and slimmer television/computer displays. The research is published in the May 26, 2005, online edition of the Journal of the American Chemical Society.
The material is a "polymer nanocomposite," meaning it consists of distinct organic polymer and inorganic regions that self-organize naturally into composite "building blocks" with dimensions on the order of a nanometer, or billionth of a meter. When put together, these basic units form the bulk material.
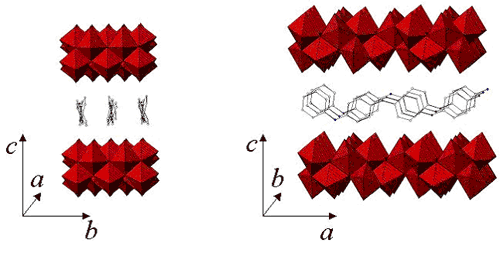
A front (left) and side view of the three-dimensional structure of the polymer nanocomposite. Two double layers of vanadium oxide molecules (red) sandwich a layer of polyaniline chains.
"Polymer nanocomposites have been attracting a lot of attention because of their potential to improve many technologies," said Brookhaven physicist Tom Vogt, who participated in the study. "The polymer imparts unique mechanical properties, such as the ability to bend and stretch, and both components are good electrical conductors."
The polymer component involved here is known as polyaniline. It forms a large family of polymer nanocomposites when combined with various inorganic compounds, such as metal oxides. In this case, the inorganic compound consists of the metal vanadium bound to oxygen atoms (vanadium oxide), separated by layers of water molecules.
Previously, the structure of this nanocomposite was not well understood, mainly because its "building blocks" are not arranged in a regular, ordered way. Thus, the group could not rely on conventional structural analysis methods that use x-rays, since those methods require a crystalline sample with a high degree of order.
In conventional x-ray diffraction, x-rays "bounce," or diffract, away from the atoms in the sample, and emerge in well defined bunches. When analyzed, the x-ray bunches yield precise information about the type and position of atoms in the sample. But when scattering x-rays off nanocomposites, the bunches are "smeared out," obscuring the structural information.
Therefore, this group used an unconventional mathematical method to interpret the x-ray scattering data. This method was first applied to data from the polyaniline polymer alone, then the vanadium oxide compound alone, and then finally to the polymer nanocomposite.
Using these separate pieces of information, the researchers were finally able to create a three-dimensional model of how polyaniline and vanadium oxide mix at the atomic level to form the polymer nanocomposite. The model depicts polyaniline chains packed horizontally with a double layer of vanadium oxide both above and below, forming a "sandwich."
"Our results demonstrate how coupling a widely used x-ray analysis technique with a non-traditional experimental approach makes it possible to obtain detailed structural information about nanocomposite materials. This improves our understanding of the materials' properties," said lead researcher Valeri Petkov from Central Michigan University. "We hope our work will stimulate more investigations of this type."
Additionally, the project involved work by Michigan State University scientist Mercuriou Kanatzidis, who, with his group, pioneered a way to synthesize these nanocomposite materials.
The research was funded by the National Science Foundation.
2005-10332 | INT/EXT | Newsroom









