Trapping Argon Atoms in Two-Dimensional Arrays of Tiny Cages
June 26, 2017
What is the scientific achievement?
CFN scientists have reversibly trapped and released single argon atoms inside tiny cages made of silicon, aluminum and oxygen. The single-atom cages are formed in a flat zeolite crystal only 0.5 nanometers thick. The molecular permeability of the 2D zeolites is reversibly tuned by the presence of argon.
Why does this achievement matter?
Trapping single gas atoms on a surface allows them to be studied using sensitive surface science probes, and provides an experimental route to obtaining unprecedented detailed, atomic-scale information about the behavior of confined gases.
What are the details?
 enlarge
enlarge
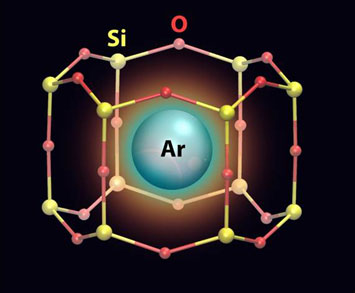
Illustration of an argon atom trapped inside a two-dimensional zeolite cage. The coordinates of the zeolite atoms calculated using density functional theory calculations.
CFN Capabilities:
The CFN/NSLS-II partner synchrotron X-ray Ambient Pressure Photoelectron Spectroscopy instrument was used to study trapping and release of argon atoms. The CFN Theory and Computation facility provided resources for calculating energetics of this process.
Publication Reference
J.-Q. Zhong, M. Wang, N. Akter, J.D. Kestell, A.M. Boscoboinik, T. Kim, D.J. Stacchiola, Deyu Lu & J.A. Boscoboinik. Immobilization of single argon atoms in nano-cages of two-dimensional zeolite model systems. Nat. Commun. 8, 16118. doi: 10.1038/ncomms16118 (2017).
Acknowledgement of Support
Research carried out at the Center for Functional Nanomaterials and National Synchrotron Light Source II, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-SC0012704.
This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
A.M. Boscoboinik was supported by CONICET, Argentina.
J.Q. Zhong and M. Wang are supported by BNL LDRD Project No. 15-010.
2017-12323 | INT/EXT | Newsroom