Engineering a Multi-Element Atomic Arrangement
A novel disorder-to-order transition strategy for ordered nanoparticles published in Science Advances
February 15, 2022
The following press release, originally issued by the University of Maryland, describes a new approach to synthesize a unique class of intermetallic compounds at the nanoscale. The research team used electron microscopes at the Center for Functional Nanomaterials (CFN) to visualize atomic ordering in nanoparticles. These nanoparticles were also heated to 1,100 degrees Kelvin (1,520 degrees Fahrenheit) inside of a CFN microscope to confirm the stability of ordered atomic structure at high temperatures. CFN is a U.S. Department of Energy (DOE) Office of Science User Facility at DOE’s Brookhaven National Laboratory. For more information on Brookhaven’s role in this research, contact Stephanie Kossman (skossman@bnl.gov, 631-344-8671).
By Katie Holland
 enlarge
enlarge
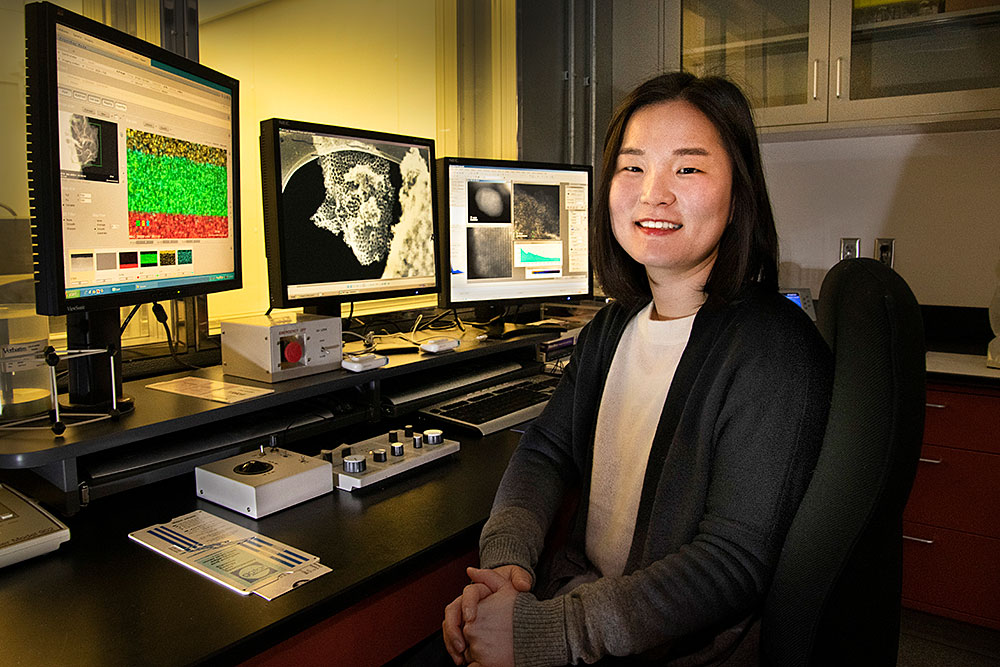
Sooyeon Hwang, staff scientist in the electron microscopy group at Brookhaven's Center for Functional Nanomaterials, is a co-author of the study.
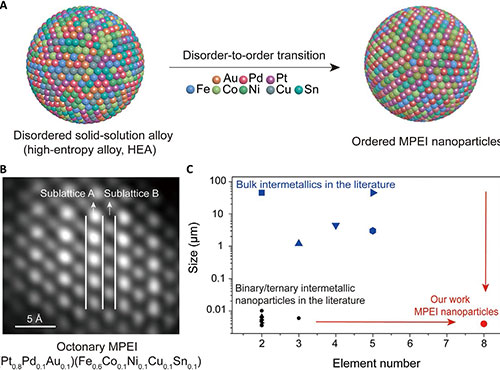
For the first time, a research group at the University of Maryland (UMD) has demonstrated the single phase nanoscale multi-principal element intermetallics (MPEIs) with up to 8 different metals – completely devoid of particle growth, or phase separation - via a novel multi-element disorder-to-order strategy. This approach demonstrates a general strategy for synthesizing MPEI nanoparticles, not only providing a step toward octonary intermetallics, but also enabling the synthesis of MPEIs at the nanoscale.
Nano-MPEIs are intermetallic compounds – roughly 4-5 nanometers diameter – composed of multi-elemental metals in definite proportions, as opposed to alloy, for example, which has variable proportions. The properties and crystal structure formed by these intermetallic compounds are different from its constituents.
The study, led by Liangbing Hu, Herbert Rabin Distinguished Professor of Materials Science and Engineering (MSE) at the University of Maryland (UMD) and director of the Center for Materials Innovation (CMI) – was published in Science Advances on January 28, 2022. Mingjin Cui, a former MSE Faculty Assistant at UMD, served as the first author on the research paper.
“Precisely controlling the atomic ordering arrangement inside nanoparticles is a great challenge,” said Hu. “We achieved the multi-elemental ordered intermetallic nanoparticles by unique disorder-to-order transition strategy. The strategy can be extended to produce a combinatorial library of intermetallic nanomaterials that may feature novel properties and applications.”
To achieve this, the group rapidly heated metal-salt precursors on a carbon substrate at 1100 K. Once cooled, they reheated the nanoparticles for five minutes, again at 1100 K, to encourage atomic rearrangement (thus ensuring the elements would fall in proper order), which gave way to a more stable MPEI arrangement. Then, the elements were rapidly cooled. The result was MPEI nanoparticles boasting an intermetallic structure with multiple elements.
“What makes this work special is the nano-MPEIs in this case demonstrate high activity and stability in catalysis,” said Cui. “Nano-MPEIs have not been achieved previously by traditional wet chemistry or long-time sintering processes.”
Not only can this process bolster applications in catalysis, magnetics and superconductors, but the group expects to create a library, so to speak, of nanoparticle MPEIs, which could potentially give way to a new frontier of intermetallic research and applications.
This multi-institutional study also included research teams from Brookhaven National Laboratory, University of Delaware, Oak Ridge National Laboratory, Dalhousie University, Washington State University and UC San Diego.
For additional information: Cui, M., Hu, L. et al. (2022). Multi-principal elemental intermetallic nanoparticles synthesized via a disorder-to-order transition, Science Advances. DOI: 10.1126/sciadv.abm4322
About the A. James Clark School of Engineering
The University of Maryland’s A. James Clark School of Engineering is a premier program, ranked among the top 20 in the world. Located just a few miles from Washington, D.C., the Clark School is at the center of a constellation of high-tech companies and federal laboratories, offering students and faculty access to unique professional opportunities.
Our broad spectrum of academic programs, including the world’s only accredited undergraduate fire protection engineering program, is complemented by a vibrant entrepreneurial ecosystem, early hands-on educational experiences, and participation in national and international competitions.
The Clark School is leading research advancements in aerospace, bioengineering, robotics, nanotechnology, disaster resilience, energy and sustainability, and cybersecurity. From the universal product code to satellite radio, SMS text messaging to the implantable insulin pump, our students, faculty, and alumni are engineering life-changing innovations for millions. Learn more at www.eng.umd.edu.
2022-19432 | INT/EXT | Newsroom