Using Enzymes to Selectively Form C-C Bonds
Scientists find a new way to rapidly construct molecules for possible applications in the pharmaceutical and agrochemical industries
October 12, 2022
 enlarge
enlarge
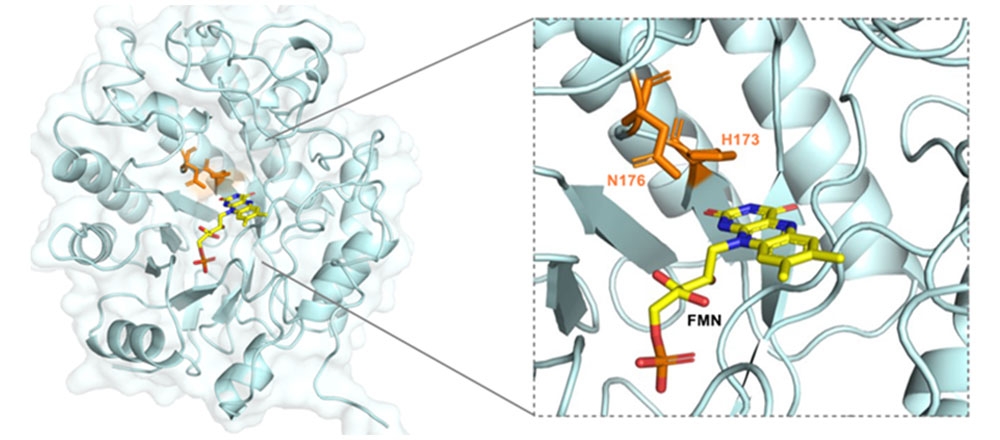
Overall crystal structure and active site of wild-type CsER (PDB code: 7TNB). Residues H173 and N176 responsible for substrate binding and the flavin mononucleotide (FMN) cofactor are labeled. Image credit: H. Fu et. al. Nature 610, 302–307 (2022).
The Science
Scientists use an enzyme to form unique carbon-carbon (C-C) bonds with unprecedented selectivity that is not possible with traditional metal-catalyzed small-molecule chemistry.
The Impact
The ability to precisely control the construction of new molecules enables the synthesis of new drugs for the pharmaceutical industry.
Summary
Cross-coupling reactions occur when two fragments are joined together to form a new carbon-carbon (C-C) bond. Transition metal-catalyzed cross-couplings have revolutionized organic synthesis, enabling the rapid construction of new molecules for the pharmaceutical and agrochemical industries. As target compounds become more complex, methods for selectively forming unique C-C bonds are needed. Specifically, coupling two distinct Csp3 electrophiles with high cross-selectivity and stereoselectivity continues as an unmet challenge.
This work reports a highly chemoselective and enantioselective cross-coupling reaction between alkyl halides and nitroalkanes that is catalyzed by flavin-dependent ‘ene’-reductases (EREDs).
The X-ray crystal structure of the flavin-bound enzyme was determined using the Highly Automated Macromolecular Crystallography (AMX) beamline at the National Synchrotron Light Source II (NSLS-II). NSLS-II is a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory. Combining docking studies and atomic resolution structural data from the active site helped the team understand the reaction mechanism.
Results showed that photoexcitation of the enzyme-templated charge-transfer complex between an alkyl halide and a flavin cofactor enables the chemoselective reduction of alkyl halide over the thermodynamically favored nitroalkane partner. The key C–C bond-forming step occurs by means of the reaction of an alkyl radical with an in-situ-generated nitronate to form a nitro radical anion that collapses to form nitrite and an alkyl radical. An enzyme-controlled hydrogen atom transfer (HAT) affords high levels of enantioselectivity. This reactivity is unknown in small-molecule catalysis and highlights the potential for enzymes to use new mechanisms to address long-standing synthetic challenges.
Download the research summary slide (PDF)
Contact
Todd K. Hyster
Cornell University
thyster@cornell.edu
Publications
H. Fu, J. Cao, T. Qiao, Y. Qi, S.J. Charnock, S. Garfinkle, T.K. Hyster. An asymmetric sp3–sp3 cross-electrophile coupling using ‘ene’-reductases. Nature 610, 302–307 (2022). [DOI: 10.1038/s41586-022-05167-1]
Funding
The authors acknowledge P. Jeffrey for assistance with X-ray structure determination and the staff of NSLS-II beamline AMX (17-ID-1) for help with data collection. The research reported here was supported by the NIH National Institute of General Medical Sciences (R01GM127703). This work made use of the Cornell University NMR Facility, which is supported, in part, by the NSF through MRI award CHE-1531632. This research used the AMX beamline at the National Synchrotron Light Source II, a United States Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. The Life Science Biomedical Technology Research resource, which supports AMX, is primarily supported by the NIH (NIGMS) through a Biomedical Technology Research Resource P41 grant (P41GM111244), and by the DOE Office of Biological and Environmental Research (KP1605010).
2022-21016 | INT/EXT | Newsroom









