New Class of RAS Inhibitors Could Treat Many Cancers
November 30, 2024
 enlarge
enlarge
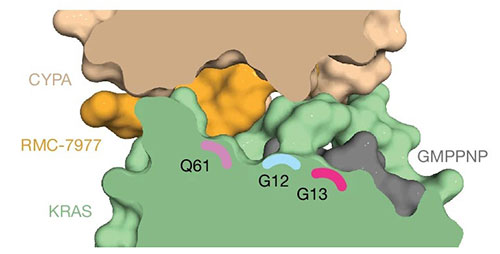
The tri-complex binding mode of RMC-7977 creates a groove between CYPA, KRAS, and RMC-7977 along the Q61–G12–G13 axis. Credit: Nature 629, 919-926 (2024)
The Science
Researchers describe the RAS(ON) multi-selective inhibitor RMC-7977, which targets the frequently mutated RAS oncogenes in tumors.
The Impact
This preclinical work supports evaluation of a new therapeutic approach for many "RAS-addicted" cancers.
Summary
A collaboration of researchers has discovered a promising inhibitor of RAS oncoproteins, which are frequently mutated in human cancers to drive tumor growth. The inhibitor, known as RMC-7977, inhibits the active form (RAS(ON)) of both wild type and mutant RAS proteins. Mutations in the RAS oncogenes — most commonly in the KRAS gene — play a role in the three deadliest cancers in the U.S.: non-small cell lung cancer, colorectal cancer, and pancreatic ductal adenocarcinoma. The group's work to detail the structure and behavior of RMC-7977 supports the evaluation of new therapies for many patients who have these cancers.
The researchers learned that RMC-7977 binds to the abundantly expressed cellular protein cyclophilin A, remodeling its surface to form a high-affinity interface with active RAS. The resulting tri-complex disrupts RAS signaling by blocking RAS interaction with effector proteins that drive tumor growth. They studied this complex in a variety of ways, including investigating its structure using x-ray diffraction at the Frontier Microfocusing Macromolecular Crystallography (FMX) beamline at National Synchrotron Light Source II (NSLS-II). NSLS-II is a U.S. Department of Energy (DOE) Office of Science user facility located at DOE's Brookhaven National Laboratory.
In preclinical cancer models, treatment with RMC-7977 led to tumor regression, including in models of cancers that are resistant to certain KRAS inhibitors. This work supports clinical evaluation of RAS(ON) multi-selective inhibitors.
Download the research summary slide (PDF)
Contact
Jacqueline Smith
Revolution Medicines
jan@revmed.com
David Wildes
Revolution Medicines
pete@revmed.com
Mallika Singh
Revolution Medicines
msingh@revmed.com
Publications
Matthew Holderfield, Bianca J. Lee, Jingjing Jiang, Aidan Tomlinson, Kyle J. Seamon, Alessia Mira, Enrico Patrucco, Grace Goodhart, Julien Dilly, Yevgeniy Gindin, Nuntana Dinglasan, Yingyun Wang, Lick Pui Lai, Shurui Cai, Lingyan Jiang, Nicole Nasholm, Nataliya Shifrin, Cristina Blaj, Harshit Shah, James W. Evans, Nilufar Montazer, Oliver Lai, Jade Shi, Ethan Ahler, Elsa Quintana, Stephanie Chang, Anthony Salvador, Abby Marquez, Jim Cregg, Yang Liu, Anthony Milin, Anqi Chen, Tamar Bar Ziv, Dylan Parsons, John E. Knox, Jennifer E. Klomp, Jennifer Roth, Matthew Rees, Melissa Ronan, Antonio Cuevas-Navarro, Feng Hu, Piro Lito, David Santamaria, Andrew J. Aguirre, Andrew M. Waters, Channing J. Der, Chiara Ambrogio, Zhengping Wang, Adrian L. Gill, Elena S. Koltun, Jacqueline A. M. Smith, David Wildes & Mallika Singh.
Nature Volume 629, pages919–926 (2024). DOI: 10.1038/s41586-024-07205-6
Funding
J.E. Klomp is funded by National Cancer Institute grants T32CA009156, F32CA239328 and K99CA276700, and American Cancer Society grant PF-20-069. P.L. is supported in part by the NIH/NCI (1R01CA23074501, 1R01CA23026701A1 and 1R01CA279264-01), The Pew Charitable Trusts, the Damon Runyon Cancer Research Foundation, and the Pershing Square Sohn Cancer Research Alliance. P.L. is also supported by the Josie Robertson Investigator Program and the Support Grant-Core Grant program (P30 CA008748) at Memorial Sloan Kettering Cancer Center. D.S. is funded by AECC Excellence Program 2022 (EPAEC222641CICS). A.J.A. has research funding from Bristol Myers Squibb, Deerfield, Eli Lilly, Mirati Therapeutics, Novartis, Novo Ventures, Revolution Medicines and Syros Pharmaceuticals. A.M.W. was supported by a grant from the NCI (K22CA276632-01). C.J.D. has received research funding support from Deciphera Pharmaceuticals, Mirati Therapeutics, Reactive Biosciences, Revolution Medicines, and SpringWorks Therapeutics, the National Cancer Institute (P50CA257911 and R35CA232113), Department of Defense (W81XWH2110692), and Pancreatic Cancer Action Network (22-WG-DERB). C.A. is funded by grants from the Giovanni Armenise–Harvard Foundation, the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101001288) and AIRC under IG 2021–ID. 25737 project. The authors acknowledge G. Verdine and scientists at WarpDrive Bio for their early work on natural product analogues as synthetic tri-complex inhibitors. The authors thank R. Zhao and K. Muonio for technical support for the LC–MS experiments; colleagues at GenenDesign, Pharmaron, Wuxi AppTec, Beijing Vital River/VR Laboratory Animal Co., Beijing AniKeeper Biotech, Shanghai Sino-British SIPPR/BK Laboratory Animal Co. and The Jackson Laboratory for technical expertise and conducting experiments; R. Bagni, M. Holderfield, D. Soppet and the US Department of Health and Human Services for supplying the engineered MEF cell lines; and K. Olive, S. Lowe and R. Bernards for constructive feedback and discussions.
2024-22228 | INT/EXT | Newsroom