A New Route to Highly Complex, Stable Metal-Organic Frameworks
April 9, 2025
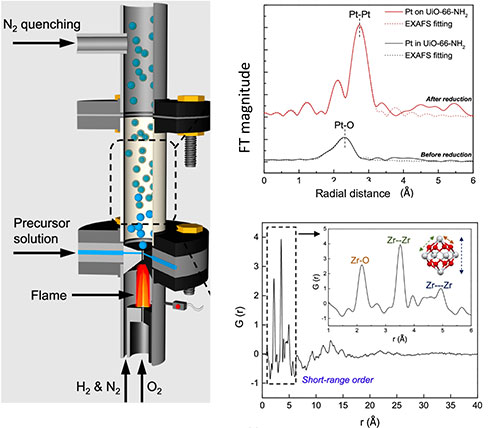 enlarge
enlarge
(A) Visualization of the MOF synthesis process, (B) Extended X-ray Absorption Fine Structure (EXAFS) spectra recorded for Pt/MOF at the ISS beamline, and (C) Pair Distribution Function (PDF) recorded at the XPD beamline for amorphous MOF.
The Science
Compared to conventional synthesis methods, where synthesis takes hours, the materials are synthesized within milliseconds — drastically increasing scalability.
The Impact
A faster process for creating complex MOFs that allows accelerated applications in catalysis, biomedicine, and more. The synthesis method is scalable for mass production. Results showed 100% CO conversion at 130°C.
Summary
Metal-organic frameworks (MOFs) are highly porous and structurally flexible materials that are being studied for a wide range of potential applications, from biomedicine to sensors to catalysis. In this study, a group of researchers describes a new, efficient way to synthesize high quality, elementally complex MOFs with a range of chemistries.
Most MOFs are relatively simple in terms of composition. But here, the group was able to integrate different metal ions within a single MOF framework, even when not thermodynamically favorable. They also found that dopant metals, such as platinum and palladium, can be removed from the MOF framework by reduction, forming nanoclusters anchored on the MOF. This feature could be particularly useful in catalysis.
The approach they used is a flame aerosol process, a type of synthesis that is often used in industry to create powders. It typically involves introducing a precursor material — in this case, a liquid of solvent, metal ions, and organic linker molecules — to a steady flame. Within the flame, the precursors undergo chemical reactions and form nanoparticles.
In this case, the researchers modified the flame aerosol process so that it used milder heating, adding precursors outside of the flame, which was important to preserve the organic linker molecules. They were able to create two distinct classes of nanomaterials: MOFs with nanocrystalline structures and MOFs with amorphous structures. Both classes have advantages. Nanocrystalline MOFs have a high surface-area-to-volume ratio, which means more active sites and smaller distances between the micropores of the MOF structure — good for catalysis. Amorphous MOFs lack long-range order but tend to have enhanced thermal stability, flexibility, optical properties, and ion conductivity — traits that are valuable for many applications.
The MOF structures were comprehensively characterized by a range of techniques. These included X-ray absorption spectroscopy at the Inner Shell Spectroscopy beamline at the National Synchrotron Light Source II (NSLS-II) and pair distribution function analysis at NSLS-II's X-ray Powder Diffraction beamline.
NSLS-II is a U.S. Department of Energy (DOE) Office of Science user facility located at DOE’s Brookhaven National Laboratory.
Download the research summary slide (PDF)
Related Links
A general flame aerosol route to kinetically stabilized metal-organic frameworks
Contact
Chaochao Dun
Lawrence Berkeley National Laboratory
cdun@lbl.gov
Jeffrey J. Urban
Lawrence Berkeley National Laboratory
jjurban@lbl.gov
Mark T. Swihart
University at Buffalo, The State University of New York
swihart@buffalo.edu
Publications
Shuo Liu, Chaochao Dun, Feipeng Yang, Kang-Lan Tung, Dominik Wierzbicki, Sanjit Ghose, Kaiwen Chen, Linfeng Chen, Richard Ciora, Mohd A. Khan, Zhengxi Xuan, Miao Yu, Jeffrey J. Urban, Mark T. Swihart. A general flame aerosol route to kinetically stabilized metal-organic frameworks. Nat Commun 15, 9365 (2024). DOI: https://doi.org/10.1038/s41467-024-53678-4
Funding
This work at the University at Buffalo (SUNY) was supported by the DOE National Energy Technology Laboratory under Grant number DE-FE0032209. Work at the Molecular Foundry at Lawrence Berkeley National Laboratory was supported by the Office of Science, Office of Basic Energy Sciences of the U.S. DOE under Contract No. DE-AC02-05CH11231. This research used resources of the 8-ID (ISS) and 28-ID-2 (XPD) beamlines of the National Synchrotron Light Source II, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract no. DE-SC0012704.
2025-22482 | INT/EXT | Newsroom









