Improving Next Gen Solid-State Lithium Batteries with Halide Separation
August 13, 2025
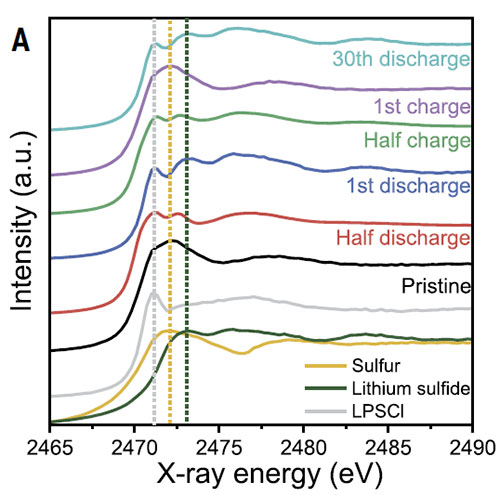
Sulfur K-edge XANES spectra of the 5-hour ultra high speed-mixed composite S/LPSCl/C cathode at different discharge and charge states.
The Science
Researchers discovered that mixing battery components at ultra-high-speed causes lithium halide compounds to form a protective layer around cathode particles in all-solid-state lithium-chalcogen batteries.
The Impact
This fast, scalable technique improves performance, enabling a long cycle life at room temperature without extra coatings, paving the way for safer, cost-effective, longer-lasting solid-state batteries.
Summary
When compared to current lithium-ion batteries, all-solid-state lithium-sulfur batteries are a safer alternative with a higher energy density. However, current all-solid-state battery technology does not use sulfur efficiently and has challenges achieving a long cycle life. In this collaborative study, researchers from Argonne National Laboratory and The National Synchrotron Light Source II, a U.S. Department of Energy Office of Science user facility at DOE’s Brookhaven National Laboratory, and The Advanced Light Source, a U.S. DOE Office of Science user facility at Lawrence Berkeley National Laboratory, demonstrated that when cathode materials are mixed with halogen-containing solid electrolytes at 2000 rpm for several hours it causes lithium halide compounds to naturally form a thin, protective layer around the cathode particles in all-solid-state lithium-chalcogen batteries. This process, called halide segregation, greatly improves how ions move through the battery and cushions the expansion and contraction of sulfur, keeping the battery structure intact through hundreds of charge cycles. Batteries with this treatment showed almost 100% sulfur utilization and retained up to 93% of their capacity after 450 cycles.
The team used several advanced tools, including cryo-electron microscopy and several synchrotron X-ray techniques, to watch the halide layer form and see how it improved ion flow and structure. At NSLS-II, multimodal characterization was performed including X-ray diffraction characterization at the X-ray Powder Diffraction (XPD) and X-ray absorption spectroscopy at the Tender Energy X-ray Absorption Spectroscopy (TES) and Inner-Shell Spectroscopy (ISS) beamlines.
This simple, scalable technique dramatically increases battery performance, allowing for a significantly greater sulfur utilization and improved cycling stability at room temperature in commercial-level capacities. It eliminates the need for complex coatings or additives and works across a range of cathode materials, including sulfur, selenium, and tellurium, offering a pathway toward safer, longer-lasting, cost-effective solid-state batteries for large scale applications like electric vehicles and more.
Download the research summary slide (PDF)
Related Links
https://www.science.org/doi/10.1126/science.adt1882
Contact
Khalil Amine
Argonne National Laboratory
amine@anl.gov
Gui- Liang Xu
Argonne National Laboratory
xug@anl.gov
Publications
J. Lee, S. Zhou, V. C. Ferrari, C. Zhao, A. Sun, S. Nicholas, Y. Liu, C. Sun, D. Wierzbicki, D. Y. Parkinson, J. Bai, W. Xu, Y. Du, K. Amine, G.L. Xu. Halide segregation to boost all-solid-state lithium-chalcogen batteries. Science 388,724-729(2025). DOI:10.1126/science.adt1882
Funding
Research at Argonne National Laboratory was funded by the DOE’s Vehicle Technologies Office. Funding support from S. Thompson and T. Duong of the DOE’s Vehicle Technologies Office program is gratefully acknowledged.
2025-22585 | INT/EXT | Newsroom









