BNL Researchers Debut New Glass Reactor to Study Battery Materials Synthesis
Path to defect-free lithium iron phosphate will improve performance
September 29, 2011
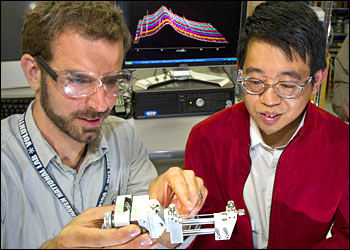
Jason Graetz, left, and Jiajun Chen at NSLS beamline X14 A
A team of Brookhaven researchers has fabricated a new transparent chemical reactor vessel that may give scientists in many fields a window into real-time chemistry. Scientists in the Lab’s Energy Storage Group recently used the transparent reactor to study the synthesis of lithium iron phosphate for rechargeable batteries. The technique, described in a paper published recently in the Journal of Physical Chemistry Letters, allowed them to monitor the reactions in real time and pinpoint the conditions for producing a defect-free material.
Eliminating defects from materials used in lithium ion batteries is essential to making them attractive for applications such as electric vehicles capable of driving hundreds of miles on a single charge. They are already favored compared to other rechargeables for portable electronics because they are lightweight, can store more energy for longer periods of time, and can handle more cycles of use and recharge without deteriorating.
A stumbling block for their use in larger applications like electric vehicles has been cost. A big part of that cost comes from processing the lithium iron phosphate material to produce a suitable, defect-free material using the conventional synthesis method.
“We wanted to identify the mildest conditions necessary to make defect-free lithium ion phosphate,” said Brookhaven Materials Scientist Jason Graetz, leader of the Energy Storage Group.
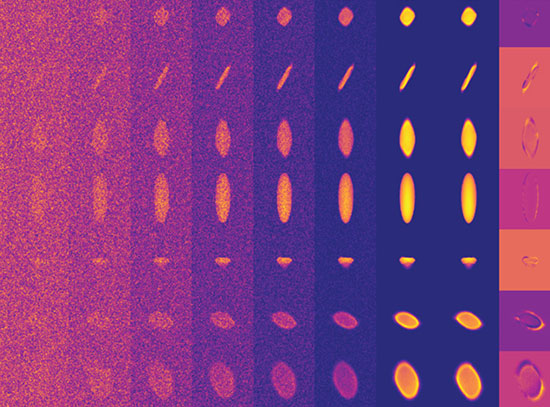
“Generally we make battery materials in a stainless steel reactor. There’s no window, no way to see the reaction — we just see what goes in and what comes out. So we designed a reactor made out of a glass capillary and, using synchrotron x-ray diffraction, we can not only probe the precursors — the initial parts of the reaction — but we can also track what happens as the reaction takes place.”
The scientists started with a slurry of both solid and liquid precursors, placed them in the glass capillary reaction vessel, and placed the whole setup in beamline X14A at the National Synchrotron Light Source (NSLS), a source of extremely bright x-rays and other forms of light for probing materials’ structure and properties. NSLS researchers Haiyan Chen and Jianming Bai helped to fabricate the novel reactor. As the x-rays pass through the transparent reaction vessel, they bounce off, or get diffracted by, the atoms in the reactor, producing a pattern that reveals the atomic structure of the various materials in the reactor and how they change as the reaction takes place.
“Because we’re getting the diffraction pattern, we can learn something about the structure,” Graetz said. “By analyzing these diffraction patterns, we can also learn about the defect concentration in the material and can track the defects in real time as a function of temperature or time in the reaction.”
By doing a series of experiments at different temperatures and different lengths of time, the scientists can identify where the defects are and where they start to disappear, allowing them to pinpoint the lowest temperature and the simplest reaction to produce a defect-free material.
“This method has proven to be a cost-effective and industrial viable method for manufacturing battery materials,” said Brookhaven Materials Scientist and lead (corresponding) author on the paper, Jiajun Chen. “We’re trying to find the relationship between the synthesis conditions — pressure, temperature, time, concentration of solution — and how that relates to the material morphology, the defect concentration, and the structure. Establishing those relationships often can take a very long time. But with this type of technique we can establish those very quickly.”
By identifying the ideal conditions for producing defect-free lithium iron phosphate, this research should eliminate the need for further processing, thus reducing the cost of the most expensive part of lithium-ion batteries.
This battery research is just one example of a problem that can be tackled with the new transparent reactor. Other candidate materials for batteries as well as for other applications can be made and tested more quickly than by conventional methods. When reactions can be observed in real time, researchers can save both time and money while developing needed 21st century technologies in energy and other important fields.
This work was funded through the Laboratory Directed Research and Development (LDRD) program.
2011-2561 | INT/EXT | Newsroom