NSLS Study Opens a Window into Molecular Self-Assembly
January 8, 2012
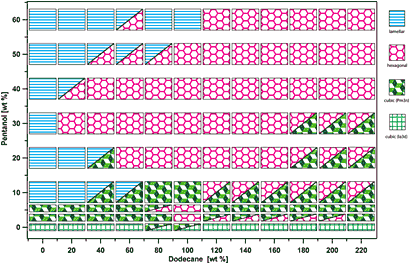
Phase diagram of ESA complexes at 100 mM NaCl salt concentration as a function of pentanol and dodecane content relative to the surfactant weight [wt %].
The natural phenomenon of self-assembly — how molecules or other entities gather to become ordered objects or arrays — occurs in many areas of science, from nanomaterials to biology. Resulting from basic, well understood forces, such as electrostatics, self-assembly may allow scientists to manipulate materials at at tiny scales, ultimately yielding great advances in fields such as data storage, pharmaceuticals, and catalysis.
In efforts to better understand self-assembly, many researchers are studying self-assembling systems in solution that consist of an electrolyte (any molecule that dissociates into ions in solution) attached to, or “in complex with,” a surfactant, a substance that lowers the surface tension between two liquids (or between a liquid and a solid). These liquid systems, called polyelectrolyte-surfactant complexes (PSCs), are good platforms for scientists to study how polymers and small molecules bind and also how they behave in solution.
Looking ahead, scientists are targeting PSCs as a route toward creating functional materials with specific jobs, such as nanostructures for drug delivery. But investigating PSC behavior can be difficult because the complexes self-assemble into phases of many different structures, including cubic assemblies of various-sizes, hexagonal assemblies of cylinders, and stacks. Scientists want to find a method that will allow them to “dial in" a preferred phase.
In a significant step toward this ability, researchers working at NSLS recently mapped out the full phase diagrams of a new, enhanced type of PSC, determining all the possible phases given different solution concentrations. The group, which consists of researchers from Stony Brook University, NSLS, and the University of Massachusetts Amherst, call their system an electrostatically self-assembled amphiphilic complex, or ESA.
“My lab is interested in creating ordered nanoporous materials for applications in filters, catalysis and fuel-cell membranes,” said the study’s corresponding scientist, Helmut Strey from Stony Brook University. “This published work is the first step towards this goal by employing self-assembly to create porous materials with tunable pore size.”
In PSC systems, the different phases are dictated mainly by spherical clusters of surfactant molecules, called micelles, which form when the surfactant is in solution. The phases are governed by the micelles’ size and curvature, and how “bendy" they are, and thus these are the characteristics the researchers need to control.
At NSLS, the group discovered that adding a cosurfactant called pentanol to the mix allows the surfactant micelles to loosen up. By then incorporating large amounts of an oily liquid called dodecane, the researchers caused each phase's unit cell (the building block of the structure) to nearly double in size. This degree of manipulation is a big step forward in the drive to understand self-assembly and use it to create new materials and technologies.
Said Strey, “The advantage of self assembly is that to make a material you just have to throw all the ingredients together and the material forms by itself. In the future, we will develop techniques to solidify our structures to create usable nanoporous materials.”
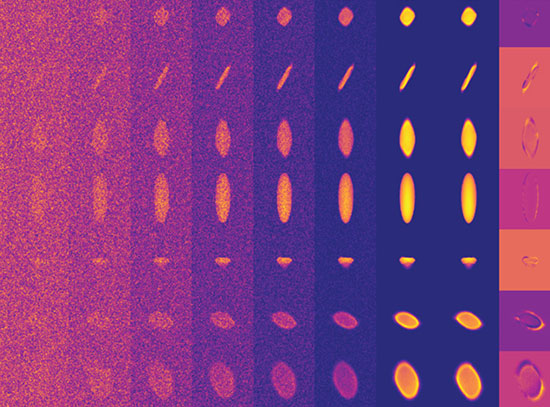
The scientists used x-rays to study the system in its various concentrations, employing an advanced technique that allows them to study many samples at once. This high throughput is achieved using a well plate scanner, a sample analysis system that combines a well plate — a tool that can hold many samples at once via with an array of small wells — with a motorized stage that can hold up to three well plates at a time and pass them through the x-ray beam. Using this method scientists can study up to 1,000 samples per day.
“This development is exciting because of the possibilities beyond this research,” said co-author Elaine DiMasi, NSLS physicist and group leader for the Soft Matter Interface beamline at the future National Synchrotron Light Source II. “As beams become smaller and data acquisition becomes faster, potentially every dataset becomes an 'image,' whether we map out a sample in real space, in 'chemical composition' space, like this work, or in processing space, such as by changing temperature or applying mechanical stress.
“Once we can render such data in automatic ways, we have a way to 'light up' regions of interest even in very large parameter spaces, and quickly hone in on the properties of interest to our applications.”
At NSLS-II, the third-generation light source that will succeed NSLS, the extremely bright x-ray beams produced will enable researchers to increase throughput, approaching a rate of one sample per second!
This research is described in the August 25, 2011, online edition of Macromolecules.
Summary Slide (.pdf)
2012-2808 | INT/EXT | Newsroom