Converting Methane to Methanol—With and Without Water
Studies of common copper-zinc oxide catalyst suggest strategies for improving water-free conversion
November 8, 2021
 enlarge
enlarge
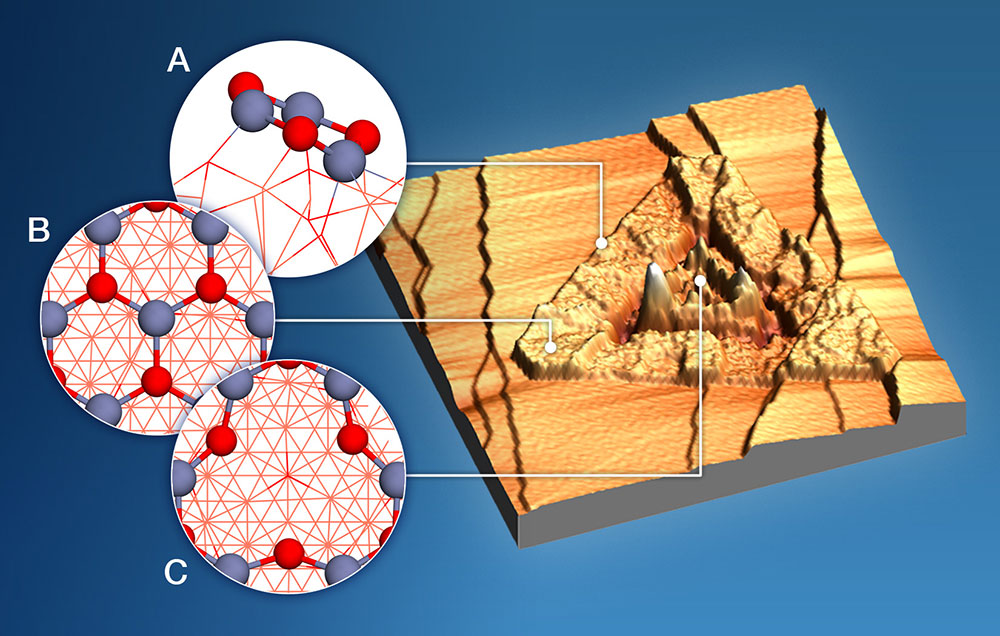
This scanning tunneling electron microscope image shows the structure of a copper-zinc oxide catalyst that converts methane to methanol with and without water. Triangular zinc-oxide "islands" rest on a copper-oxide thin film (flat background) over a copper substrate (not seen). The "step" edges between copper-oxide and zinc-oxide (A, where blue is zinc, red is oxygen) are the main active sites for producing methanol when no water is present. The semi-flat areas have a relatively perfect crystal structure (B) and are inert during the reaction. The very rough areas are likely associated with defects—in this case with fewer zinc atoms and an oxygen-rich crystal structure (C)—and are the most active sites for methanol production when water is present.
UPTON, NY— Chemists have been searching for efficient catalysts to convert methane—a major component of abundant natural gas—into methanol, an easily transported liquid fuel and building block for making other valuable chemicals. Adding water to the reaction can address certain challenges, but it also complicates the process. Now a team at the U.S. Department of Energy’s Brookhaven National Laboratory has identified a new approach using a common industrial catalyst that can complete the conversion effectively both with and without water. The findings, published in the Journal of the American Chemical Society, suggest strategies for improving catalysts for the water-free conversion.
“Water is like a trick that people have been using for a long time to get this reaction going—and it definitely helps. It improves the selectivity and it aids the ability to extract the methanol,” said José Rodriguez, a leader of Brookhaven Lab’s Catalysis Group, who has an adjunct appointment at Stony Brook University (SBU) in the departments of Chemistry and Materials Science and Chemical Engineering.
As shown in a recent related study by this group, adding water keeps the reaction from running away to transform the desired product, methanol, into carbon monoxide (CO) and carbon dioxide (CO2). But adding water also adds complexity and cost. Plus, at the temperatures and in the amounts required for this reaction, the water exists as large quantities of steam, which would have to be controlled in an industrial setting.
So, the Brookhaven team set out to explore if they could run the reaction without water by changing the catalyst—the substance that brings the reactants together and helps guide them along a particular reaction pathway.
 enlarge
enlarge
Some members of the research team (l to r: José Rodriguez, Erwei Huang, Ping Liu, Ivan Orozco, and Sanjaya Senanayake) in front of Brookhaven Lab's Chemistry Building.
Catalytic conversion
The new paper describes how a common copper-zinc oxide catalyst can steer the reaction along different pathways depending on whether water is present.
“Copper-zinc oxide is a commercial catalyst that is readily available and not too expensive,” said Sanjaya Senanayake, one of the study co-authors. “We wanted to see whether it might work for methane-to-methanol conversion.”
According to their study results, copper-zinc oxide has the best selectivity of any catalyst tested for this reaction without the addition of water—about 30%. That means methanol, the desired product (instead of CO or CO2), makes up 30% of the products of the reaction when it runs without water. (When run with water, the copper-zinc oxide catalyst had 80% selectivity for methanol production.)
For comparison, the team’s earlier studies of this reaction using a cerium oxide catalyst produced almost no methanol without water.
“One of the big challenges of this methanol synthesis reaction in the presence of just methane and oxygen (and no water) is overoxidation—the transformation of the methanol into carbon monoxide and carbon dioxide,” said study co-author Ping Liu. She noted how the earlier studies of the cerium catalyst revealed how water helped to block that overoxidation by removing the produced methanol before it could be further transformed.
To find out how the copper-zinc catalyst achieves 80 and 30 percent specificity with and without water, respectively, the team conducted detailed studies using a variety of techniques that worked hand-in-hand with theoretical calculations to reveal crucial details of the reaction mechanism.
X-ray studies
“Two of our SBU graduate students, Ivan Orozco and Feng Zhang, and one of our postdocs, Zongyuan Liu, worked with Slavomír Nemšák, a long-time collaborator, at the Advanced Light Source (ALS)—a DOE Office of Science user facility at Lawrence Berkeley National Laboratory—to find evidence of methanol formation on the surface of the catalyst,” Senanayake said. “The technique, called ‘ambient-pressure x-ray photoemission spectroscopy (XPS),’ uses ALS’s bright beams of x-rays to detect the carbon, hydrogen, oxygen, and the metal-oxygen combinations at the active sites of the catalyst as the reaction is taking place.
The scientists studied the samples under different reaction conditions. They varied the amount of methane, oxygen, and water (including no water), as well as the pressure and temperature—tracking which chemical species were present at different stages of the reaction.
"Water is like a trick that people have been using for a long time to get this reaction going."
– Brookhaven Lab chemist José Rodriguez
“Each compound has a unique ‘chemical fingerprint,’ so we can see how these reactants are transformed into intermediates and final products under different conditions,” Rodriguez said.
The XPS fingerprints clearly showed that methanol was forming. But to find out exactly which sites on the catalyst were involved in the reactions, the team turned to theoretical modeling.
Modeling atomic interactions
The team used a scanning tunneling microscope in Brookhaven’s Chemistry Department to study the atomic-level structure of the catalyst, and then used those structural details to build computational models of the atomic arrangements.
“There are many diverse active sites on the surface of the catalyst,” said Liu. To understand those sites and determine whether and how they interacted with the reactants and products, another SBU graduate student—Erwei Huang—and Liu ran “density functional theory” (DFT) calculations and kinetic modeling on computing clusters at Brookhaven Lab’s Center for Functional Nanomaterials (CFN) and the National Energy Research Scientific Computing Center (NERSC) at Lawrence Berkeley National Laboratory.
DFT calculations identify how the reactants (methane, oxygen, and water) evolve as they interact with one another and the catalyst, as well as how much energy it takes to get from one atomic arrangement to the next. Kinetic modeling tries out all the possible pathways for those transformations to take place under reaction conditions.
This combination of techniques allowed the team to identify the most energy-efficient (and therefore most likely) path for how methane is transformed into methanol with and without water. The results included details about which catalytic sites were involved and which intermediates should be present at different stages during the reaction. The team then verified these catalytic interactions and intermediates with “chemical fingerprinting” measurements at ALS.
Pathways to improvement
Together, the data indicate that the reaction proceeds along two different pathways involving two different sites of the copper-zinc-oxide catalyst—one for the reaction with water and one for the reaction without water.
“The particular configuration of active sites for the reaction with water is different from the configuration without water, and the mechanism is different, too—it’s practically two different processes,” Rodriguez said.
But in both cases, even without water, “the binding between the methanol and the catalyst is strong enough to allow the methanol to form from methane, but weak enough to enable the methanol to come off the surface as a gas before it is further oxidized to CO or CO2,” Liu said.
“As soon as the methanol goes into the gas phase you can condense the whole thing and then separate liquid methanol,” Rodriguez said.
That quick “desorption” of methanol from the surface of the catalyst, which keeps the methanol from reacting further with oxygen, also eliminates a potentially explosive step.
The team is already using their new knowledge of the reaction mechanisms to look for ways to further improve the catalyst. Their goal is to achieve a selectivity of at least 60-70% without water.
“The atomic level understanding is much more advanced than what we’ve ever had before. We know really atom by atom that copper zinc oxide is much better for the preferred no-water reaction condition,” Senanayake said.
In the next step, DFT calculations and kinetic modeling will start to test out other compositions, aiming to further improve the methane conversion and methanol selectivity.
“We’ll use the theory to narrow down the candidates based on the mechanistic understanding acquired from the previous studies,” Liu said. “Then the experimentalists will do the synthesis and characterization studies to see if these other compositions will work.”
This research was funded by the DOE Office of Science (BES). ALS, CFN, and NERSC are DOE Office of Science user facilities.
Brookhaven National Laboratory is supported by the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://www.energy.gov/science/.
Follow @BrookhavenLab on Twitter or find us on Facebook.
2021-19139 | INT/EXT | Newsroom