Scientists Use AI and X-ray Vision to Gain Insight into Battery Electrolyte
Artificial intelligence and experimental validation reveal atomic-scale basis for improved 'water-in-salt' battery performance
May 22, 2025
 enlarge
enlarge
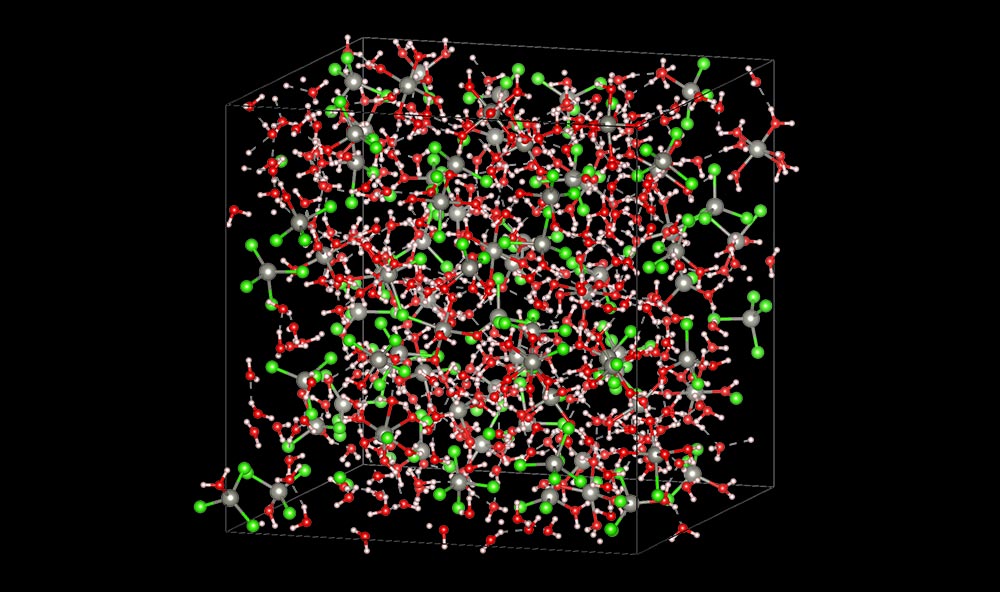
Scientists used AI to model how zinc and chloride ions (gray and green spheres) at different concentrations would interact with and move through water (oxygen and hydrogen represented by red and white spheres) in an aqueous battery electrolyte. The AI-assisted modeling revealed that a high concentration of zinc chloride salt solution stabilizes water in the electrolyte while maintaining sufficiently high conductivity — characteristics that are essential for aqueous zinc-ion battery performance. (Chuntian Cao/Brookhaven National Laboratory)
UPTON, N.Y. — A team of scientists from the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Stony Brook University (SBU) used artificial intelligence (AI) to help them understand how zinc-ion batteries work — and potentially how to make them more efficient for future energy storage needs. Their study, published in the journal PRX Energy, focused on the water-based electrolyte that shuttles electrically charged zinc ions through the rechargeable battery during charging and use. The AI model tapped into how those charged ions interact with water under varying concentrations of zinc chloride (ZnCl2), a form of salt with high solubility in water.
The AI findings, validated by experiments at Brookhaven Lab’s National Synchrotron Light Source II (NSLS-II), show why high salt concentrations produce the best battery performance.
“AI is an important tool that can facilitate the advancement of science,” said Esther Takeuchi, chair of the Interdisciplinary Science Department (ISD) at Brookhaven Lab and the William and Jane Knapp Chair in Energy and the Environment at SBU. “The research done by this team provides an example of the insights that can be gained by combining experiment and theory enhanced by the use of AI.”
Amy Marschilok, manager of the Energy Storage Division of ISD and a professor of chemistry at SBU, added, “This work could help advance the development of robust zinc-ion batteries for large-scale energy storage. These batteries are particularly attractive for resilient energy applications because the water-based electrolyte is inherently safe and the materials use to make them are abundant and affordable.”
Water in salt
Like all batteries, zinc-ion batteries convert energy from chemical reactions into electrical energy, explained Deyu Lu, a staff scientist in the Theory and Computation Group of Brookhaven Lab’s Center for Functional Nanomaterials (CFN) who led this research.
“However, competing chemical reactions, such as those that split water molecules and produce hydrogen gas, can severely degrade battery performance,” he said. “If any of this energy is used in side reactions, you lose energy that is supposed to do work.”
Lu and his collaborators knew that previous studies had found that water splitting is suppressed in a special zinc chloride electrolyte where the salt concentration is so high it’s referred to as “water-in-salt,” in contrast to more common “salt-in-water” electrolytes. To figure out why the high-salt version was better, they wanted to capture the atomic-scale details of how zinc and chloride ions move and interact with water — and how that affects the electrolyte’s conductivity — at different salt concentrations.
But seeing these atomic-scale details is extremely challenging. So the team turned to a form of computer modeling enhanced by AI vision.
 enlarge
enlarge
Study co-authors Deyu Lu, Chuntian Cao, and Amy Marschilok analyze AI-generated images that model electrolyte performance in the Theory and Computational Facility at Brookhaven Lab's Center for Functional Nanomaterials. (Kevin Coughlin/Brookhaven National Laboratory)
Developing AI vision
“Seeing these complex details would be impossible using conventional computing techniques,” Lu said. “Conventional simulation methods cannot handle the large number of atomic interactions with the desired accuracy to capture the timescales over which such systems evolve. Such calculations require enormous computing power, which would easily take many years.”
So instead of performing all the complex calculations that would be needed to fully simulate the ions’ interactions with water, the team used conventional simulations to generate a small number of simulation data, known as a “training set,” and fed it to an AI program. They used computing resources at the Theory and Computational Facility at CFN, a DOE Office of Science user facility, and Brookhaven Lab’s Scientific Computing and Data Facilities within the Computing and Data Sciences directorate (CDS).
“We needed a little bit of data collected by calculating a small number of interactions to kickstart the process of training an initial model,” said CDS’s Chuntian Cao, first author on the paper. “Then, we ran the model to generate more data to continue to improve the model’s predictions.”
At each step, the scientists ran their results through an ensemble of machine learning (ML) models to assess whether the predictions were accurate. Lu likened the process to calling several friends to help answer questions on “Who Wants to be a Millionaire,” a once-popular TV game show. “If the friends/models all agree, then it looks like you have good chance that you have an accurate prediction,” he noted.
But, as Cao pointed out, “When we find that some predictions have very large deviations in the ensemble of ML models, we return to doing the conventional calculations to get the correct answer. These new corrected data points are then added back to the training data to further refine the ML model.”
This iterative “active learning” process minimized the number of calculations that needed to be run in a computationally expensive way to complete the training of the ML model. And, after several rounds of training, the AI model could make predictions about much larger numbers of atomic interactions over longer and longer timescales.
“Chuntian ran the simulations with several thousands of atoms, a very large system, for hundreds of nanoseconds — an impossible task using the conventional methods. AI/ML is truly a game changer in the study of complex materials,” Lu said.
Stablizing water
The Brookhaven and Stony Brook scientists’ AI model revealed that high zinc chloride concentrations play the key role in stabilizing water molecules, protecting them from splitting.
In pure water, the oxygen atom in one water molecule (H2O) forms two so-called hydrogen bonds with hydrogen atoms in neighboring water molecules. These hydrogen bonds connect the water moleclues in a continuous network that makes the water molecules more reactive and susceptible to splitting, Lu said.
The team found that the number of hydrogen bonds drops rapidly as the zinc chloride concentration increases, disrupting the hydrogen-bond network. In the water-in-salt regime, only about 20% of the hydrogen bonds are left.
“Stabilizing the water molecules is an essential component of why high-concentration water-in-salt electrolytes work so well,” said Cao.
 enlarge
enlarge
Study co-authors Esther Takeuchi, Shan Yan, and Milinda Abeykoon at the Pair Distribution Function beamline of the National Synchrotron Light Source II, where "X-ray mapping" experiments revealed structural details that validated AI predictions about electrolyte properties. (Kevin Coughlin/Brookhaven National Laboratory)
Shuttling zinc
But electrochemical stability isn’t the only benefit of water-in-salt electrolytes revealed by this study. The AI model also provides an explanation for how the high salt concentration maintains efficient zinc ion transport.
“When your battery is cycling, your ion is going back and forth between the electrodes. You want these ions to be mobile; you don't want them to be locked up,” Lu noted.
The AI model revealed that at very low concentrations, the zinc and chloride ions are separated from one another and move through the electrolyte independently in opposite directions, due to their opposite charges, Lu explained. At higher concentrations, the ions and water molecules start to form clusters with a net negative charge. This overall negative charge makes these zinc clusters move in the wrong direction compared to the preferred direction for positively charged zinc ions. “This is really bad,” said Lu.
Fortunately, at very high concentration, some zinc, chloride, and water aggregates grow very large, “like icebergs,” Lu said. Though still negatively charged, there are very few of these large clusters, so they contribute little to conductivity. But smaller clusters left in the solution acquire an overall positive charge and can zip around the big clusters to provide high enough conductivity for the battery to work.
Validating experiments
The scientists didn’t completely rely on the agreement among the ML models to assess their results. They also did real-world experiments to study the atomic structures and measured the electrical conductivity of electrolyte samples.
At NSLS-II, a DOE Office of Science user facility, the scientists used X-rays at the Pair Distribution Function (PDF) beamline to generate measurements of the distribution of distances between pairs of atoms in the material.
“The PDF beamline provides a powerful platform with adjustable X-ray energies that give a direct picture of how atoms are spaced,” said study coauthor Milinda Abeykoon, the lead scientist for the beamline. “This high-resolution X-ray mapping helps researchers explore structures ranging from just a few atoms to much larger patterns, which is especially useful for studying complex materials like those found in batteries. It’s a great way to cross-check and validate atomic-level structures predicted by machine learning methods.”
Study coauthor Shan Yan of ISD said, “These measurements provide us with information about the solvation structure of ions, which can be very important to understanding how the electrolyte functions.”
The AI-based predictions agreed well with the real-world experiments. “So, we are confident that the model is reliable,” Cao said.
“This work demonstrates the great impact artificial intelligence and machine learning can have for understanding the chemistry of materials and provides guidelines for optimizing battery electrolytes,” said Lu. “It represents a strong collaboration of multiple Brookhaven Lab departments and highlights Brookhaven Lab’s unique strength in conducting interdisciplinary research that leverages large DOE Office of Science user facilities.”
In addition, Marschilok pointed out the important close coupling of theory and experiment, as well as the contributions of SBU graduate students who helped prepare samples, conduct experiments, and analyze the data.
“Working hand in hand with these graduate students and all the scientists at Brookhaven gave us a great opportunity to get the best quality of experimental data and analysis — and to train the next generation workforce in using these advanced techniques,” she said.
The work was funded by the DOE Office of Science and the National Science Foundation. The research team included scientists from CFN, CDS, ISD, the Condensed Matter Physics and Materials Science Department, and NSLS-II, along with the Department of Materials Science and Chemical Engineering, the Department of Chemistry, and the Institute of Sustainability, Electrification and Energy at Stony Brook University. Scientists from Princeton University and Temple University also contributed to the work.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
2025-22451 | INT/EXT | Newsroom