Scientists Develop Fuel-Cell Catalyst for Heavy-Duty Vehicles
New recipe for catalysts could dramatically improve performance and durability of fuel cells for powering trucks
September 24, 2025
 enlarge
enlarge
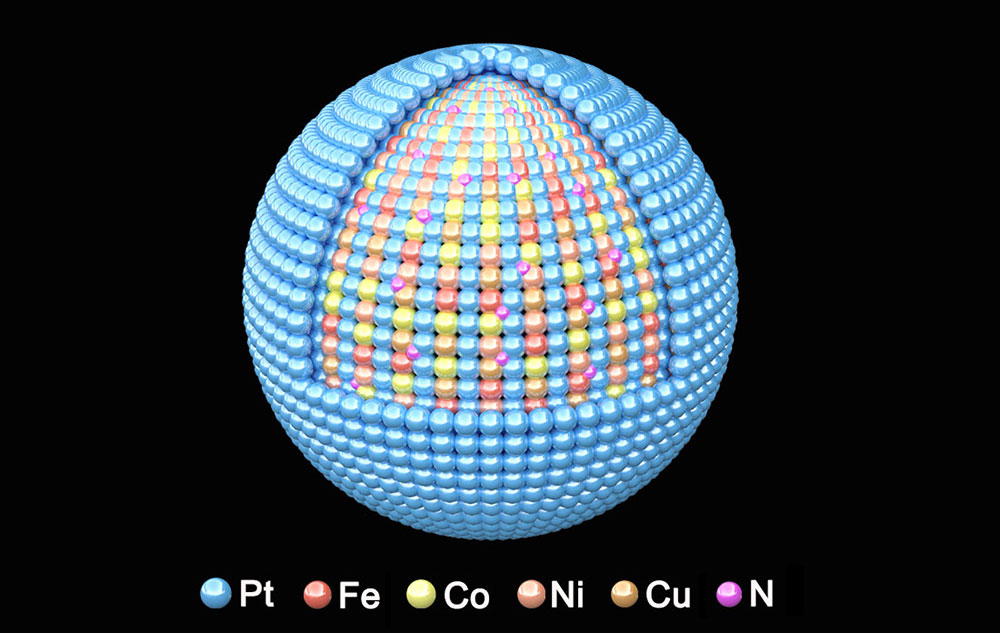
A multidisciplinary group at Brookhaven National Laboratory revealed why a catalyst with a "high-entropy" intermetallic core — composed of platinum (Pt), iron (Fe), cobalt (Co), nickel (Ni), and copper (Cu) doped with nitrogen (N) — encapsulated by a single-layer shell of Pt shows promise for use in fuel cells for heavy duty vehicles. (Brookhaven National Laboratory)
UPTON, N.Y. — Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have discovered a new recipe for catalysts that could dramatically improve the performance and durability of fuel-cell vehicles — especially trucks and other heavy-duty vehicles that demand extra power. The research, published in Nature Communications, marks a major step toward making hydrogen fuel-cell technology viable for commercial fleets, where the need for long-range, high-power solutions is greatest.
New generation of catalysts
Fuel cells are devices that can generate electricity from a variety of fuels. When fuel cells use hydrogen and oxygen, the only byproduct is water. While today’s fuel cells work well in passenger cars, there is growing interest in using fuel cells to efficiently power heavy-duty vehicles like buses, freight trucks, and long-haul transport vehicles. One of the biggest challenges has been designing catalysts that are tough and efficient enough to handle the extra demands of such heavy-duty applications.
“Catalysts are the components that enable the chemistry at the electrodes inside a fuel cell,” said Kotaro Sasaki, the Brookhaven chemist who led the research along with Xueru Zhao, also of the Chemistry Division. “These materials, often made of metals, bring the reacting chemicals together and lower the energy required to run the reaction. But the catalyst has to be able to perform this function over and over in challenging conditions, such as high heat or a harsh acidic environment. Our study focused on designing a catalyst that can sustain high performance in these challenging conditions.”
The Brookhaven-led team designed a new nitrogen-doped catalyst made from a carefully tuned mix of five metals: platinum, cobalt, nickel, iron, and copper. Such “high-entropy intermetallic” materials — so named because they contain multiple elements within an ordered atomic structure — are especially stable, making them promising candidates for use in extreme environments.
By zooming in on the material at the atomic level using X-rays and microscopy at Brookhaven’s National Synchrotron Light Source II (NSLS-II) and Condensed Matter Physics and Materials Science Department, the researchers discovered that the new catalyst has tiny distortions in its atomic structure, partly caused by what they refer to as “sub-angstrom strain.” These subtle shifts in atomic position — measuring much less than the width of a single atom — combined with strong bonds between the metals and nitrogen atoms, make the catalyst both highly active and unusually durable.
 enlarge
enlarge
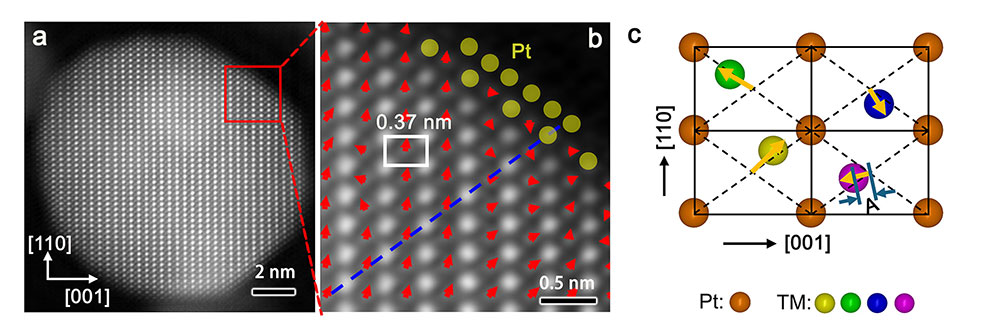
High-resolution transmission electron microscopy revealed the core–shell structure of the catalyst, including how the Pt monolayer shell protects the multimetallic core from leaching. Sub-angstrom displacements of transition-metal (TM) atoms within the core (indicated by red arrows in image b and yellow arrows in image c) provide direct evidence of intrinsic lattice strain, a key factor that boosts the catalyst's stability under fuel cell operating conditions. (Brookhaven National Laboratory)
Record-setting performance
In rigorous tests simulating heavy-duty truck operation, the new catalyst maintained excellent performance over 90,000 operating cycles — which is equivalent to continuous operation for 25,000 hours — producing current densities well above DOE targets. This achievement was part of a DOE initiative to advance fuel cell technologies to enable their commercialization for use in heavy-duty vehicles and other applications.
“These results show a practical pathway to building fuel-cell systems that can power the trucks and buses of tomorrow,” said Zhao. “Our catalyst not only meets immediate market needs but also lays the groundwork for widespread adoption in heavy-duty transportation.”
Collaboration across the Lab
This work was made possible by close collaborations across multiple Brookhaven Lab research groups. Scientists from the Surface Electrochemistry and Electrocatalysis group in the Chemistry Division contributed expertise in synthesis and electrochemistry, while teams in the Condensed Matter Physics and Materials Science Department and the Center for Functional Nanomaterials (CFN) carried out advanced materials characterization. Researchers at NSLS-II, one of the world’s most advanced light sources, provided critical insights into the structure of the new catalyst using the Quick X-ray Absorption and Scattering (QAS) and In situ and Operando Soft X-ray Spectroscopy (IOS) beamlines.
“This is a clear example of how fundamental research at a national laboratory can have real-world impact,” said Zhao. “By uncovering the atomic-scale mechanisms that make this catalyst so effective, we’re opening the door to practical technologies that meet industry needs.”
This work was funded by the U.S. Department of Energy. CFN and NSLS-II are DOE Office of Science user facilities at Brookhaven Lab.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
2025-22607 | INT/EXT | Newsroom