Confirmation of Covalent Bonding in α-Pu
August 13, 2025
 enlarge
enlarge
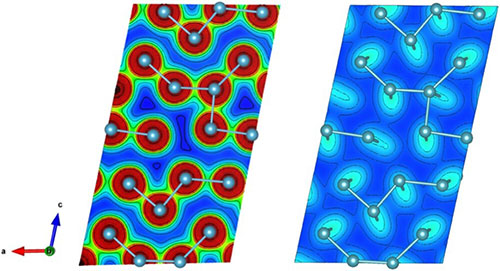
The charge structure of α-Pu. (Left) The charge density in the atomic plane shows distinct signatures of bonding exist between neighboring atoms at short bond lengths. (Right) The charge density on the interatomic plane shows less charge between neighboring atoms.
The Science
Scientists uncover joint experimental and theoretical evidence of covalent bonding in α-plutonium (α-Pu) for the first time.
The Impact
Atomistically understanding how different types of bonding influence Pu allotrope behaviors could reshape how these materials are used for nuclear energy applications, like batteries and reactors.
Summary
Plutonium is famous for its use in nuclear technology, but its internal structure, especially in its alpha-phase (α-Pu), has puzzled scientists for decades due to its complex bonding behavior and unusual properties. With the advancement of scientific tools within the field, these long unanswered questions can finally be answered. In a recent collaborative effort between Los Alamos National Laboratory and The National Synchrotron Light Source II, a U.S. Department of Energy Office of Science user facility at DOE’s Brookhaven National Laboratory, advanced computer simulations and high-precision X-ray measurements were able to uncover how atoms bond in α-Pu for the first time.
By combining high-energy X-ray pair distribution function (PDF) measurements at NSLS-II’s PDF beamline with advanced theoretical modeling using density functional theory (DFT), the team’s analyses revealed that α-Pu hosts a mix of bonding types: short bonds exhibit directional, covalent-like character, while longer bonds behave more metallically. This mixed bonding landscape aligns with the theory that α-Pu’s structure is shaped by a Peierls distortion, when a material slightly changes the positions of its atoms to lower its overall energy.
The presence of covalent bonding helps explain why α-Pu behaves more like a brittle solid than a malleable metal. This study marks the first time experimental data and modern theory have been used together to confirm covalent bonding in α-Pu. The results open new doors to understanding how atomic structure shapes the properties of complex, radioactive materials that can be used in energy applications like nuclear batteries and reactors.
Download the research summary slide (PDF)
Related Links
https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adfm.202501798
Contact
Alexander Muñoz
Los Alamos National Laboratory
alexmunoz@lanl.gov
W. Adam Phelan
Los Alamos National Laboratory
wap@lanl.gov
Publications
R. Muñoz, M. S. Cook, D. C. Arellano, A. M. Abeykoon, J. N. Mitchell, S. C. Hernandez, E. D. Bauer, C. A. Mizzi, B. Maiorov, N. Harrison, W. A. Phelan, Experimental and Theoretical Confirmation of Covalent Bonding in α-Pu. Adv. Funct. Mater. 2025, 2501798. https://doi.org/10.1002/adfm.202501798
Funding
All authors gratefully acknowledge funding for this work under LANL-LDRD projects 20210001DR, 2023004DR, and 20220538ECR. Further, all authors are grateful to Tomas Martinez, Carlos Archuleta, Christopher Cordova, Derek V. Prada, William S. Ponder, and Paul H. Tobash for sample coordination and preparation. This research used resources provided by the Los Alamos National Laboratory Institutional Computing Program, which is supported by the U.S. Department of Energy National Nuclear Security Administration under Contract No. 89233218CNA000001. Use of the National Synchrotron Light Source-II, Brookhaven National Laboratory, was supported by the U.S. Department of Energy under Contract No. DE-SC0012704.
2025-22584 | INT/EXT | Newsroom









