Brain-Behavior Disconnect in Cocaine Addiction
Impaired ability to monitor behavior, emotions may underlie vulnerability to drugs; suggests new targets for treatment
May 25, 2009
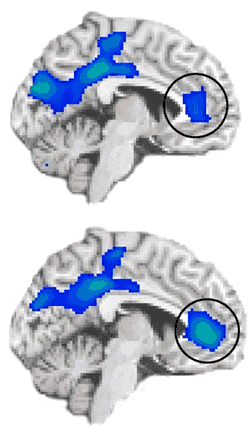
During an emotionally salient task, fMRI scans of cocaine-addicted individuals (bottom) showed stronger deactivation in the anterior cingulate cortex compared with scans of healthy controls (top). This deactivation suggests that this region may have been trying to "tune out" emotional stimuli, such as craving.
UPTON, NY — Parts of the brain involved in monitoring behaviors and emotions show different levels of activity in cocaine users relative to non-drug users, even when both groups perform equally well on a psychological test. These results — from a brain-imaging study conducted at the U.S. Department of Energy’s Brookhaven National Laboratory and published online the week of May 25, 2009, by the Proceedings of the National Academy of Sciences — suggest that such impairments may underlie addictive vulnerability, and that treatments aimed at improving these functions could help addicted individuals resist drugs.
“Many studies have found decreased brain activity in drug-addicted individuals relative to healthy control subjects during psychological tests,” said lead author Rita Goldstein, a psychologist at Brookhaven Lab. “But it’s never been clear if these differences were due to varying levels of interest or ability between the two groups. This is the first study to look at two groups matched for performance and interest — and we still see dramatic differences in the brain regions that play a very significant role in the ability to monitor behavior and regulate emotion, which are both important to resisting drug use.
“Whether these brain differences are an underlying cause or a consequence of addiction, the brain regions involved should be considered targets for new kinds of treatments aimed at improving function and self-regulatory control,” Goldstein said.
The researchers studied 17 active cocaine users and 17 demographically matched healthy control subjects. Both groups were trained to push one of four colored buttons corresponding to the color of type used to present words that were either related to drug use (e.g., crack, addict) or neutral household terms. Subjects were given monetary rewards for fast, accurate performance — up to 50 cents for each correct answer on some tests, for a maximum of $75.
After training, both groups performed equally well on this same test while lying in a magnetic resonance imaging (MRI) scanner, with performance improving when they knew they’d be earning the highest monetary reward. During the tests, the scientists used functional MRI (fMRI) to indirectly measure the amount of oxygen being used by specific regions of the brain, as an indicator of brain activity in those regions.
There were three main differences between the cocaine-addicted subjects and the healthy controls:
- The cocaine users had reduced activity in a portion of the anterior cingulate cortex that usually becomes more active (compared to a passive baseline) when monitoring behavior. Activity levels were lowest during the least “interesting,” or salient, version of the test — when there was no monetary reward and the words shown were neutral household terms. Within the cocaine-user group, activity levels were lowest in the people who had used cocaine most frequently in the 30 days prior to the test.
- The cocaine users also had reduced activity in another part of the anterior cingulate cortex that usually becomes less active (compared to a passive baseline) when someone is successfully suppressing emotional feelings. Within the cocaine-user group, activity levels during the high-salience version of the test — when each fast, correct answer was rewarded with 50 cents and the words presented were drug-related — were lowest in the people who were most successful in suppressing the task-induced craving. In healthy controls, who did not report craving, activation in this region was not significantly different from baseline.
- The functions within the behavior-monitoring and emotion-monitoring brain regions were interconnected in the healthy control subjects but not in the addicted individuals. In all, these group differences in brain function and interconnectivity were quite robust and all the more meaningful in that there were no differences between the groups in performance on or interest ratings for the task.
“When you really have to suppress a powerful negative emotion, like sadness, anxiety or drug craving, activity in this brain region is supposed to decrease, possibly to tune out the background ‘noise’ of these emotions so you can focus on the task at hand,” Goldstein said.
“Our results show that activity in this region indeed went down in the drug-using group, suggesting they were actively trying to suppress craving. Indeed subjects who reported the highest levels of task-induced craving were the least able to suppress activity in this particular brain region.
“This could be because these drug users were still being distracted by background ‘noise’ stimuli, like memories of having taken drugs or anticipation of further use,” Goldstein said.
“This work gives us some clues as to what happens when drug users are unable to suppress craving — and how that might work together with a decreased ability to monitor behavior, even during neutral, non-emotional situations, to make some people more vulnerable to taking drugs,” Goldstein said.
The findings point to the importance of improving activity in the behavior-monitoring brain region, possibly by using behavioral and pharmacological approaches to increase motivation and top-down monitoring. Treatments aimed at strengthening activity in the emotion-monitoring brain region may further help addicted individuals regain self-control, especially during hard to suppress highly emotional situations (e.g., during craving). Treatments aimed at strengthening the interconnectivity between these brain regions may decrease impulsivity.
This study was supported by grants from the National Institute on Drug Abuse and the General Clinical Research Center of Stony Brook University.
Brookhaven Lab has a world-renowned research program aimed at understanding the neurological mechanisms and consequences of drug addiction. This program is fueled in part by the Department of Energy’s long-standing support of brain-imaging technologies such as MRI and positron emission tomography (PET), which were developed as a direct outgrowth of DOE’s commitment to basic physics and chemistry research.
2009-10957 | INT/EXT | Newsroom










