New Source of Brilliant Deep Blue Light
Copper-iodide thin films form foundation for efficient and stable LEDs with hard-to-achieve deep blue hues
July 16, 2025
By Karen McNulty Walsh and Kitta MacPherson
 enlarge
enlarge
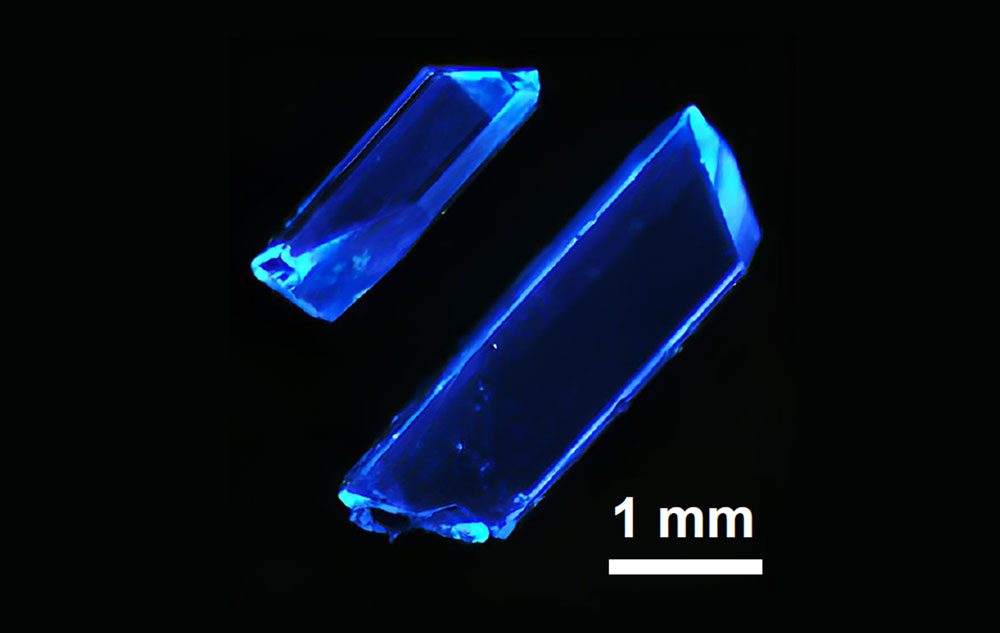
A microscopic image of hybrid copper iodide crystals emitting deep-blue light developed by scientists at Rutgers University (Kun Zhu/Rutgers University)
UPTON, N.Y. — Despite the apparent brilliance of display screens that light up the world, deep blue hues are hard to come by. Now, a team of scientists led by Rutgers University that includes researchers at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory has developed an approach for manufacturing light-emitting diodes (LEDs) that emit deep blue light with a wavelength of about 450 nanometers. As described in a paper just published in Nature, these deep-blue LEDs made from abundant, eco-friendly materials could improve the performance of solid-state lighting and displays.
“Deep-blue LEDs are at the heart of today’s energy-efficient lighting technologies,” said Jing Li, a professor at Rutgers who led the study. “However, existing options often present issues with stability, scalability, cost, efficiency, or environmental concerns due to the use of toxic components. This new copper-iodide hybrid offers a compelling solution, leveraging its nontoxicity, robustness, and high performance.”
LEDs are lighting devices that use special materials called semiconductors to turn electricity into light in an efficient and durable way. Blue LEDs were discovered in the early 1990s and earned their discoverers the 2014 Nobel Prize in Physics. Blue LEDs are particularly important because they are used in combination with red and green LEDs to create white light and are essential for general lighting applications.
Li and her colleagues at Rutgers collaborated with scientists from Brookhaven and four other research teams representing national and international institutions in the effort to work on new materials that would improve upon existing blue LEDs. They found a way to make blue LEDs more efficient and sustainable by using a new type of hybrid material: a combination of copper iodide with organic molecules.
“Our processing method minimizes defects that can impede the movement of electric charges at the interface of these hybrid materials,” said Kun Zhu, a former graduate student and postdoctoral associate at Rutgers who is now at the Max Planck Institute in Germany and is the paper’s first author. “This approach could be a versatile strategy for generating high-performance LEDs.”
Nanoscale studies
To characterize the LED materials’ properties and the mechanism responsible for their enhanced performance, the Rutgers team turned to long-time collaborators at Brookhaven Lab’s Center for Functional Nanomaterials (CFN). This DOE Office of Science user facility houses specialized optical tools including ultrafast spectroscopy and microscopy facilities that allow scientists to track how electric charges move through materials at very fast timescales, and over very short distances measured in billionths of a meter.
 enlarge
enlarge
Mircea Cotlet, manager of the Advanced Optical Facility at Brookhaven National Laboratory's Center for Functional Nanomaterials (Joseph Rubino/Brookhaven National Laboratory)
“This work fits perfectly with CFN’s mission of making these tools accessible to our partners so we can understand, for example, how different synthesis and processing methods change the nanoscale properties of materials and affect their performance,” said Mircea Cotlet, a co-author on the paper who manages the Advanced Optical Facility at CFN. “Importantly, these tools give us unique access to the interfaces between different materials, which are often crucial to how devices function.”
Hybrid LEDs, for example, can be imagined as a sandwich with different layers, where each layer has a specific job, such as emitting light or transporting electrons. Sometimes, the emissive layer doesn't interact perfectly with its interface layers, which can reduce efficiency or shorten lifespan.
To minimize those issues, the Rutgers team developed an innovative technique called dual interfacial hydrogen-bond passivation. This technique forms hydrogen bonds between the layers to create better connections.
Studies at Brookhaven Lab’s CFN helped reveal why this approach boosts performance.
“We used a technique called femtosecond pump-probe transient absorption spectroscopy, which is dedicated to measuring charge injection, charge transfer, and charge carrier dynamics,” Cotlet said. In essence, this technique uses a state-of-the-art laser amplifier to visualize how the electrons move through the material. And, as Cotlet noted, “We can combine that with microscopy to be able to look at very small areas of the interface between the components of the hybrid material.”
Having different ultrafast techniques within one laboratory gives scientists the opportunity to probe different stages of the process with different methods that are nearly impossible to find in a university lab. “These kinds of detailed measurements can only be done at specialized facilities like CFN,” Cotlet said
As Kun Zhu noted, “With the aid of ultrafast spectroscopies at CFN, both the intrinsic properties of the blue emitter and the enhancement brought by interfacial hydrogen bonding were comprehensively elucidated. The time-correlated single photon counting (TCSPC) technique, highly sensitive to carrier lifetimes, clearly reflected the surface passivation effects introduced by the hydrogen bonding.”
High quantum efficiency
The new hybrid copper-iodide semiconductor offers a number of advantages over other materials used in LEDs, the scientists said. Lead-halide perovskites contain lead, which is toxic to humans, and can degrade over time due to their sensitivity to moisture and oxygen. Organic LEDs (OLEDs) are flexible and potentially efficient but can degrade and lose their color quality. Colloidal quantum dots perform well mainly in green and lower-energy LEDs and are often cadmium-based, which raises toxicity concerns. Phosphorescent organic emitters can be costly to synthesize.
 enlarge
enlarge
Kun Zhu, a former Rutgers University graduate student and postdoctoral associate, now at the Max Planck Institute in Germany (Kun Zhu)
“The new material provides an eco-friendly and stable alternative to what currently exists, addressing some of these issues and may potentially advance LED technology,” Li said.
The hybrid copper-iodide material possesses favorable qualities such as a very high photoluminescence quantum yield of about 99.6%. That is, in experimental tests where light is used as the energy input, the material converts nearly all the photoenergy it receives into emitted light. Blue LEDs made from this material have reached a maximum external quantum efficiency — the ratio between the number of emitted photons and number of electrons injected from an electrical energy source, as would be used in real-world applications — of 12.6%, among the highest achieved so far for solution-processed deep-blue LEDs.
Not only are these LEDs bright, they also last longer compared with many others. In addition, the material works well in larger-scale applications. The researchers successfully created a larger device that maintains high efficiency, showing that this material has potential to be used in real-world applications.
“Overall, this type of new material is paving the way for better, brighter and longer-lasting LEDs,” Li said.
In addition to the facilities at CFN, the researchers used resources of the Molecular Foundry and the Advanced Light Source, two additional DOE Office of Science user facilities at DOE’s Lawrence Berkeley National Laboratory, and at DOE’s National Renewable Energy Laboratory.
This work was funded by the DOE Office of Science and falls under DOE’s initiative for developing new and innovative materials and manufacturing processes that improve energy efficiency and use materials with reduced costs, particularly for power electronics.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit science.energy.gov.
Follow @BrookhavenLab on social media. Find us on Instagram, LinkedIn, X, and Facebook.
2025-22536 | INT/EXT | Newsroom