3D Protein Structure Helps in the Search for a Vaccine
May 31, 2018
 enlarge
enlarge
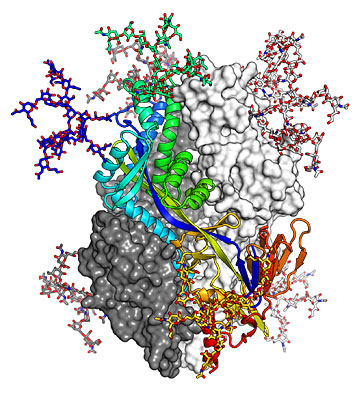
The structure of the pre-fusion hMPV F trimer is shown as ribbons and colored from blue to red, while other molecular surfaces are colored in white and dark gray. Image courtesy of Nat Commun 8, 1528 (2017).
The Science
Combining cryogenic macromolecular crystallography with modeling based on the known structure of a homologous molecule, scientists revealed the 3D structure of the human metapneumovirus (hMPV) F glycoprotein, which mediates cell-virus membrane fusion and could be a potential target for an anti-viral vaccine, with an 2.6-Å resolution.
The Impact
The hMPV is a frequent cause for bronchitis in young children, and so far no vaccine for humans has been found. In this study scientists revealed the existence of a dense glycan shield at the apex of thehMPV’s F glycoprotein. This new knowledge about the virus’ structure may help scientists in their search for a vaccine.
Summary
Scientists used cryogenic macromolecular crystallography and computer modeling based on the known structure of a homologous molecule to investigate the 3D structure of metapneumovirus F glycoprotein, which lies on the surface of human metapneumovirus (hMPV) and mediates cell-virus membrane fusion. The scientists revealed the 3D structure of the protein with a 2.6-Å resolution and found a glycan shield at the apex of the molecule.
hMPV is a frequent cause of bronchiolitis in young children, and so far no vaccine for humans has been found. The studied protein is responsible for fusion of the virus with human cells and could provide new and important information for the search for a vaccine.
It is know that the F glycoprotein rearranges its 3D structure during the fusion process with a human cells and that both arrangements have different immunological properties. While the post-fusion form of the molecule is already known, this study revealed that the pre-fusion form is significantly different from its post-fusion form. The pre-fusion form seems to possess a dense glycan shield at the apex of the molecule, which helps the virus to resist recognition by antibodies.
This study used the Highly Automated Macromolecular Crystallography (AMX) beamline at the National Synchrotron Light Source II to perform the x-ray-crystallographic measurements.
Download research summary slide
Contact
Jason McLellan
Department of Biochemistry and Cell Biology, Geisel School of Medicine at Dartmouth
Jason.McLellan@dartmouth.edu
Publications
M. Battles, V. Más, E. Olmedillas, O. Cano, M. Vázquez, L. Rodríguez, J. Melero, & J. McLellan. “Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein,” Nat Commun 8, 1528 (2017). DOI: 10.1038/s41467-017-01708-9
Funding
This research used the AMX beamline 17-ID-1 of the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. This work used electron microscopy and the Genomics core facility (ISCIII) for sequencing and was supported in part by grants 5T32AI007519-18 (M.B.B.), SAF2015-67033-R (J.A.M.), and P20GM113132 (J.S.M.). The Life Science Biomedical Technology Research resource is primarily supported by the National Institute of Health, National Institute of General Medical Sciences (NIGMS) through a Biomedical Technology Research Resource P41 grant (P41GM111244), and by the DOE Office of Biological and Environmental Research (KP1605010). As a National Synchrotron Light Source II facility resource at Brookhaven National Laboratory, work performed at the LSBR is supported in part by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences Program under contract number and DE-SC0012704 (KC0401040).
2018-13084 | INT/EXT | Newsroom









