Smaller Nanoparticles are Better Battery Materials
February 19, 2019
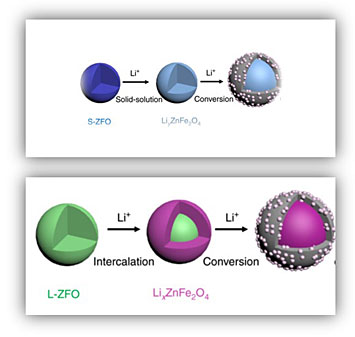
Schematic showing (top) small zinc ferrite nanoparticles forming a solid-solution during lithium insertion, followed by a conversion reaction. In contrast, (bottom) large nanoparticles follow a two-phase intercalation which is suppressed in the smaller ones.
What is the scientific achievement?
A team of scientists from the Center for Mesoscale Transport Properties Energy Frontier Research Center working with CFN staff to study in real time how the size of zinc ferrite (ZnFe2O) nanoparticles determines their performance during battery operation. The team found that the improved performance observed when using smaller nanoparticles (6-9 nm) is because lithium is taken up by forming a solid-solution, a more efficient reaction pathway.
Why does this achievement matter?
Understanding lithiation in materials as a function of particle size is helpful in designing batteries with improved performance and longevity. In-situ, dry cell transmission electron microscopy allows direct observation of structural changes in real time and with high spatial resolution.
What are the details?
Spinel transition metal oxides have emerged as promising anode materials for lithium-ion batteries. It has been shown that reducing their particle size to nanoscale dimensions benefits overall electrochemical performance. In this work, scientists from the Center for Mesoscale Transport Properties Energy Frontier Research Center worked with CFN staff using in-situ transmission electron microscopy to probe the lithiation behavior of spinel ZnFe2O4 as a function of particle size. The study showed that ZnFe2O4 undergoes an intercalation-to-conversion reaction sequence, with the initial intercalation process being size-dependent. Larger ZnFe2O4 particles (40 nm) follow a two-phase intercalation reaction. In contrast, a solid-solution transformation dominates the early stages of discharge when the particle size is about 6 to 9 nm. Using a thermodynamic analysis, the team found that the size-dependent kinetics originate from the interfacial energy between the two phases. Furthermore, the conversion reaction in both large and small particles favors {111} planes and follows a core-shell reaction mode. These results elucidate the intrinsic mechanism that permits fast reaction kinetics in smaller nanoparticles.
CFN Capabilities:
The CFN Electron Microscopy Facility was used for in-situ, ex-situ, and operando characterization.
Publication Reference
J. Li, Q. Meng, Y. Zhang, L. Peng, G. Yu, A.C. Marschilok, L. Wu, D. Su, K.S. Takeuchi, E.S. Takeuchi, Y. Zhu, E.A. Stach, Size-dependent kinetics during non-equilibrium lithiation of zinc ferrite, Nature Communications 10, 93 (2019).
DOI: 10.1038/s41467-018-07831-5
Acknowledgement of Support
This work was supported as part of the Center for Mesoscale Transport Properties, an Energy Frontier Research Center supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, under award #DE SC0012673. This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under contract No. DE-SC0012704. Q.M., L.W., and Y. Zhu are supported by U.S. DOE, DOE Office of Basic Energy Science, Division of Materials Science and Engineering, under Contract No. DE-SC0012704.
2019-14396 | INT/EXT | Newsroom









