Dual-Ion Transport in a Promising Aqueous Battery
Scientists tackle the sluggish ionic transport in aqueous batteries by revealing a new dual-ion transport mechanism
January 31, 2021
 enlarge
enlarge
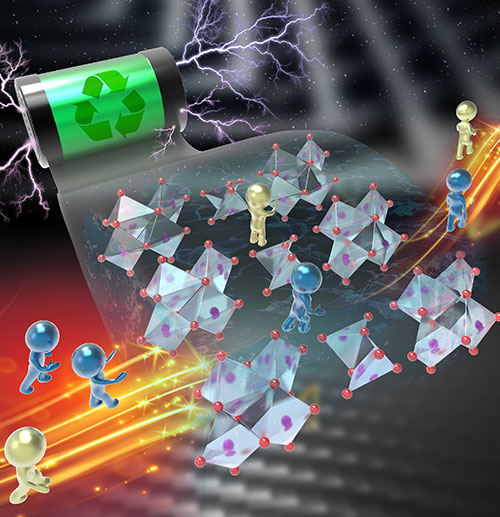
The illustration shows the dual-ion sequential storage mechanism (yellow and blue) within the structure of a sodium vanadate cathode. Image credit: Xiaoqiang Shan, Xiaowei Teng.
The Science
Scientists revealed the mechanism of dual-ion sequential storage in a sodium vanadate cathode material for a zinc-ion battery (ZIB).
The Impact
ZIBs are a promising technology for stationary, aqueous energy storage. Fully realizing this promise requires a fundamental understanding of the dual-ion sequential storage mechanism, which was given a quantitative description in this work.
Summary
Rechargeable aqueous batteries play an increasingly important role in stationary storage, which is used for applications such as wind and solar farms, especially when the cost, safety, cycling life, and power capability become more important than mere energy density. However, the current aqueous battery technology still cannot rival the storage capacity and energy density of nonaqueous lithium-ion batteries.
If scientists can address the obstacle of sluggish ionic transport in cathode materials for aqueous rechargeable zinc-ion batteries (ZIBs), these systems could compete with the capacity and energy density of nonaqueous batteries.
In this work, a team of researchers presented a quantitative analysis of a dual-ion transport mechanism found in sodium vanadate (NaV3O8) cathode materials, which can be used in ZIBs. The team investigated the structural evolution of a cathode during the charge and discharge processes using x-ray studies at the National Synchrotron Light Source II (NSLS-II), a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven Lab.
Specifically, the team used the Beamline for Materials Measurement (BMM) at NSLS-II to study the electrochemical states of the material after charging or discharging, and the Pair Distribution Function (PDF) beamline to investigate the intercalations of the ions in the material through in operando studies.
Based on their analysis, the team showed that the cathode material offers a synergetic dual-ion transport system for charge transfer, which leads to a high battery capacity and energy density, as well as a 78% retention of capacity after 2000 cycles.
The team’s work provides a comprehensive mechanistic and kinetic investigation of a sodium vanadate cathode for aqueous ZIBs. It offers new insights into developing cathodes with higher capacity and better reaction kinetics for next-generation energy storage systems through this encouraging sequential dual-ion storage mechanism.
Download the research summary slide
Contact
Xiaowei Teng
University of New Hampshire
xw.teng@unh.edu
Publication
X. Shan, S.W. Kim, A. M. M. Abeykoon, G. Kwon, D. Olds, X. Teng. Potentiodynamics of the Zinc and Proton Storage in Disordered Sodium Vanadate for Aqueous Zn-Ion Batteries. ACS Applied Materials & Interfaces 2020 12 (49), 54627-54636 DOI: 10.1021/acsami.0c15621
Funding
This work was supported by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences under Award DE-SC0018922 (X.S., S.K., and X.T.). This research used the BMM (6-BM-B) and PDF (28-ID-1) beamlines of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DESC0012704.
2021-18845 | INT/EXT | Newsroom









