New Electrolyte Could Push Li-ion Battery Performance
Scientists find a new electrolyte that addresses unwanted chemical reactions, extending battery life
September 30, 2021
 enlarge
enlarge
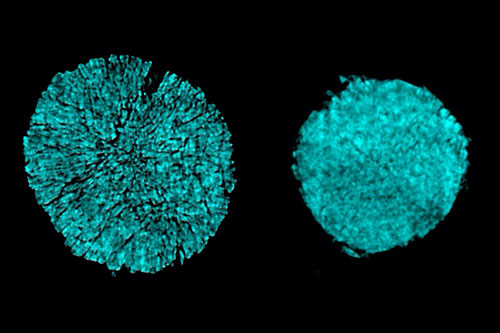
The left image shows the cracking of a particle in one electrode of a battery cell that used a conventional electrolyte, while the right shows a particle using the novel electrolyte, which prevented most of this cracking. Both images were taken at the FXI beamline at NSLS-II. Image credit: Nature Energy 6 (5), 495-505 (2021).
The Science
Scientists have found a novel electrolyte that could enable a significant leap in the power-per-weight/volume of next-generation lithium-ion (Li-ion) batteries without sacrificing the cycle life.
The Impact
Li-ion batteries are lightweight, portable power sources for many devices; however, to push their performance further, researchers needed to halt unwanted chemical and physical processes that take place under harsh electrochemical conditions. This study describes a new electrolyte that overcomes these issues.
Summary
Lithium-ion batteries are one of the most commonly used rechargeable, lightweight energy sources in the world and are a promising possibility for long-lasting power in electric vehicles. However, to push their performance to the next level, researchers need build a battery that has a higher energy density — the amount of energy that can be stored in a given mass or volume of material — than the current technologies. To achieve this, researchers have tried to use lithium metal anodes (instead of the conventional graphite) and high-voltage cathodes (based on nickel chemistry), but these efforts have been hampered by unwanted chemical and physical processes at the electrodes or within the electrolyte that separates the electrodes.
In this study, a team of researchers has found a new electrolyte that addresses both the anode and cathode challenges. One degradation effect that leads to performance degradations is the formation of cracks inside cathode particles. These cracks can occur during charging and discharging because the electrode material expands and contracts during that process.
To study how the existing electrodes would hold up when interacting the new electrolyte under electrochemical conditions, the team turned to the Full Field X-ray Imaging (FXI) beamline at National Synchrotron Light Source II (NSLS-II) to take x-ray tomography images of the electrode particles. Through these measurements, the team found that the cracking behavior is beyond a pure mechanical event and involves chemical interactions between the cathode’s surface and the electrolyte, also called “stress-corrosion” cracking. More importantly, using the new electrolyte can drastically reduce these stress-corrosion cracking degradations. The FXI beamline is part of a suite of advanced x-ray imaging tools available to researchers for various studies in materials science at NSLS-II, which is a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven Lab.
The researchers learned that the degradation is accompanied by metal atoms dissolving into the liquid electrolyte. Their new electrolyte is extremely resistant to such dissolution. This was a surprising result for the researchers because the material still readily allows lithium ions to pass through, which allows for battery charging and discharging. The team believes that their work will enable a significant leap in the power-per-weight/volume of next-generation batteries. This will, in turn, improve the performance of existing devices and enable new applications, such as long-range drones and robots.
Download the research summary slide
Contact
Yang Shao-Horn
Massachusetts Institute of Technology
shaohorn@mit.edu
Jeremiah A. Johnson
Massachusetts Institute of Technology
jaj2109@mit.edu
Ju Li
Massachusetts Institute of Technology
liju@mit.edu
Yanhao Dong
Massachusetts Institute of Technology
dongyh@mit.edu
Publications
Weijiang Xue, Mingjun Huang, Yutao Li, Yun Guang Zhu, Rui Gao, Xianghui Xiao, Wenxu Zhang, Sipei Li, Guiyin Xu, Yang Yu, Peng Li, Jeffrey Lopez, Daiwei Yu, Yanhao Dong, Weiwei Fan, Zhe Shi, Rui Xiong, Cheng-Jun Sun, Inhui Hwang, Wah-Keat Lee, Yang Shao-Horn, Jeremiah A. Johnson & Ju Li. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nature Energy 6 (5), 495-505 (2021). DOI: 10.1038/s41560-021-00792-y
Funding
We acknowledge support by the Department of Energy, Basic Energy Sciences, under award number DE-SC0002633 (Chemomechanics of Far-From-Equilibrium Interfaces). We acknowledge the cathodes provided by the US Department of Energy CAMP Facility, Argonne National Laboratory, and the LiFSI salt by KISCO. This work made use of the Material Research Science and Engineering Center Shared Experimental Facilities supported by the National Science Foundation under award number DMR-1419807. Z.S. acknowledges the research grant at the Department of Materials Science and Engineering at the Massachusetts Institute of Technology. This work used resources of the beamline FXI/18ID of the National Synchrotron Light Source II, a US Department of Energy Office of Science User Facility operated for the Department of Energy Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. This work used resources of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy Office of Science by Argonne National Laboratory, and was supported by the US Department of Energy under contract no. DE-AC02-06CH11357 and the Canadian Light Source and its funding partners. J. Lopez acknowledges support by an appointment to the Intelligence Community Postdoctoral Research Fellowship Program at the Massachusetts Institute of Technology, administered by Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the Office of the Director of National Intelligence. We also thank C. Mao at Zhu Hai Smooth Way Company for valuable suggestions and G. Leverick from Y. Shao-Horn’s group at the Massachusetts Institute of Technology for the support in measuring the water content by Karl Fisher titration.
2021-19261 | INT/EXT | Newsroom









