How Do Algae Survive Under Low Light Conditions?
Scientists show the mechanism behind low light adaptation in algae
December 14, 2022
 enlarge
enlarge
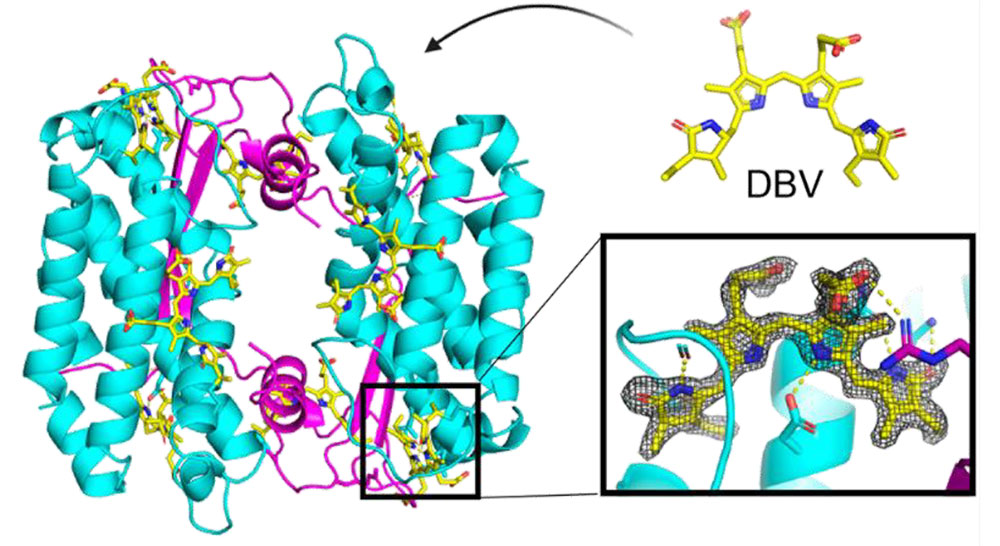
Crystal structure for photoacclimated PC577. The protein is in purple and cyan, and the chromophores are shown in yellow. There is no significant difference in crystal structure or geometry of the chromophore binding pocket from the native PC577. Photoacclimation was achieved via changes in the chromophore composition bound to the protein. ACS Cent. Sci. 8 (3), 340–350 (2022)
The Science
Scientists show that light-adaptation of certain algae occurs via chromophore tuning, and not as a result of sequence or structural changes in the light-harvesting antennae proteins.
The Impact
This research provides atomic-level insight into how algae have evolved to regulate photosynthesis under varying light conditions.
Summary
Cryptophyte algae are well-known for their ability to survive under low light conditions using their auxiliary light-harvesting antennas, called phycobiliproteins. Mainly acting to absorb light where chlorophyll cannot (500−650 nm), phycobiliproteins also play an instrumental role in helping cryptophyte algae respond to changes in light intensity through the process of photoacclimation. Until recently, photoacclimation in cryptophyte algae was only observed as a change in the cellular concentration of their phycobiliproteins; however, an additional photoacclimation response was recently discovered that causes shifts in the phycobiliprotein absorbance peaks following growth under red, blue, or green light.
In this work, scientists reproduce this newly identified photoacclimation response in two species of cryptophyte algae and examine the origin of the response at the protein level. They compared isolated native and photoacclimated phycobiliproteins using X-ray crystallography at the Highly Automated Macromolecular Crystallography (AMX) and Frontier Macromolecular Crystallography (FMX) beamlines at the National Synchrotron Light Source II (NSLS-II). NSLS-II is a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory. Combined with spectroscopy and mass spectrometry measurements, they found that neither the protein sequences nor the protein structures are modified by photoacclimation. Instead, the findings indicated that photoacclimation was achieved by changing the chromophores bound to the phycobiliproteins. This research sheds light on a newly identified cryptophyte photoacclimation process and provides a key insight into how natural systems regulate photosynthesis.
Download the research summary slide (PDF)
Contact
Gregory D. Scholes
Princeton University
gscholes@princeton.edu
Publication
L.C. Spangler, M. Yu, P.D. Jeffrey, G.D. Scholes. Controllable Phycobilin Modification: An Alternative Photoacclimation Response in Cryptophyte Algae. ACS Cent. Sci. 8 (3), 340–350 (2022). DOI: 10.1021/acscentsci.1c01209
Funding
The protein crystallography measurements of PC577 were obtained in the Protein Crystallography Core of the Molecular Biology Department at Princeton University. Measurements of PE545 were obtained using the AMX and FMX beamlines of the National Synchrotron Light Source II, a United States Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. The Center for BioMolecular Structure (CBMS) is primarily supported by the NIH, National Institute of General Medical Sciences (NIGMS) through a Center Core P30 Grant (P30GM133893), and by the DOE Office of Biological and Environmental Research (KP1605010). This work was funded by the Princeton University Writing Center and CIFAR (Canadian Institute for Advanced Research). Dr. Gregory Scholes is a CIFAR fellow.
2022-21019 | INT/EXT | Newsroom









