Multimodal X-Ray Imaging of Single Cell Reveals Complex Inner Workings
October 9, 2024
 enlarge
enlarge
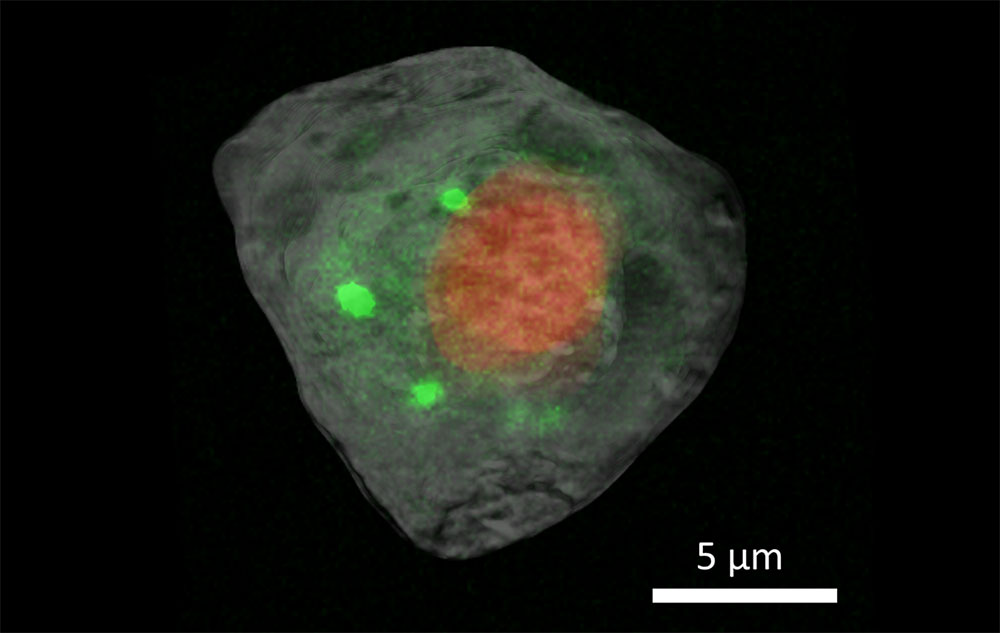
These superimposed tomography images show the nucleus (red) and cytosol (gray) with correlative X-ray fluorescence images of calcium distribution (green) in a human embryonic kidney cell.
The Science
Scientists from the U.S. Department of Energy’s Brookhaven National Laboratory have developed a novel multimodal method to image a single cell, achieving nanometer resolution without damaging delicate organelles.
The Impact
Understanding the inner workings of cells and their interactions at such detailed levels can transform how we approach various fields, particularly in identifying how pathogens interact with host cells. This multimodal imaging approach provides insights that could enhance drug delivery systems and improve agricultural practices by studying plant-pathogen interactions. These insights could also lead to better strategies for combating infectious diseases and improving crop resilience.
Summary
Cells from the human embryonic kidney (HEK) 293 line were chemically preserved using paraformaldehyde to maintain the structure of the cell. They were then rapidly frozen, transferred to liquid nitrogen, and freeze dried before being placed under a microscope to locate and label them for targeted imaging. Standardized sample holders were created for use on multiple pieces of equipment to ensure that the cell could be reliably held in one place for multiple measurements.
The team used two imaging techniques found at NSLS-II — X-ray computed tomography (XCT) and X-ray fluorescence (XRF) microscopy. They obtained the XCT data, which uses X-rays to tell scientists about the cell’s physical structure, on the Full Field X-ray Imaging (FXI) beamline. Tomography uses X-rays to show a cross-section of a solid sample. A familiar example of this is the CT scan, which medical practitioners use to image cross sections of any part of the body.
The researchers collected XRF microscopy data, which provides more clues about the distribution of chemical elements within the cell, on the Submicron Resolution X-ray Spectroscopy (SRX) beamline. In this technique, the researchers direct high energy X-rays at a sample, exciting the material and causing it to emit X-ray fluorescence. The X-ray emission has its own unique signature, letting scientists know exactly what elements the sample is composed of and how they are distributed to fulfill their biological functions.
Getting a high-resolution fluorescence map on many cells can be very time consuming. Even just for a 2D image, it may take quite a few hours. This is where getting a 3D image of the cell using XCT is helpful. This information can help guide the fluorescence measurements to specific locations of interest. It saves time for the scientists, increasing throughput, and it also ensures that the sample doesn’t need to be exposed to the X-rays for as long, mitigating potential damage to the fragile cell.
View the research summary highlight
Related Links
Paper - Correlative single-cell hard X-ray computed tomography and X-ray fluorescence imaging
Feature Article - Pioneering the Cellular Frontier
Contact
Yang Yang
Brookhaven National Laboratory
yyang@bnl.gov
Xianghui Xiao
Brookhaven National Laboratory
xiao@bnl.gov
Qun Liu
Brookhaven National Laboratory
qunliu@bnl.gov
Publications
Lin, Z., Zhang, X., Nandi, P. et al. Correlative single-cell hard X-ray computed tomography and X-ray fluorescence imaging. Commun Biol 7, 280 (2024). https://doi.org/10.1038/s42003-024-05950-y
Funding
This work was supported by Laboratory Directed Research and Development Program LDRD21-013 of Brookhaven National Laboratory and the U.S. Department of Energy (DOE), Office of Biological and Environmental Research (KP1601011). The work used the Full-field X-ray Imaging (FXI) beamline at 18-ID and the Submicron Resolution X-ray Spectroscopy (SRX) beamline at 5-ID of the National Synchrotron Light Source II, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. The work used the Vitrobot at the Laboratory for BioMolecular Structure, which is supported by the U.S. DOE Office of Biological and Environmental Research (KP1607011). The work used the confocal microscope at the Center of Functional Nanomaterials, which is a U.S. DOE Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704.
2024-22399 | INT/EXT | Newsroom









