Previously Unknown Strain-Relief Mechanism Revealed
February 11, 2026
 enlarge
enlarge
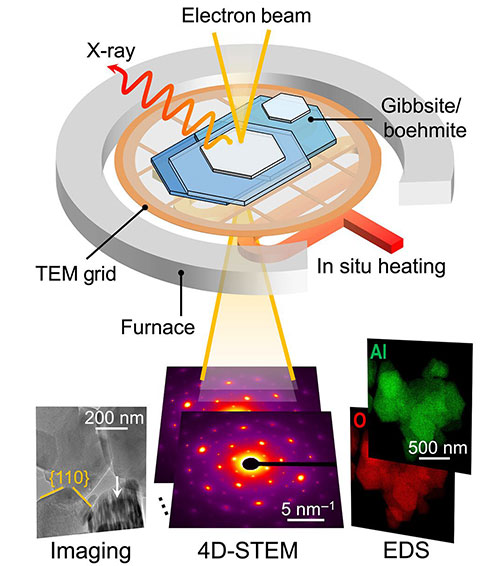
X-ray diffraction was used to resolve the gibbsite/boehmite crystal structure, while low-dose transmission electron microscopy imaging captures morphologies. In addition, 4D scanning transmission electron microscopy combined with energy-dispersive spectroscopy mapping reveals nanoscale local heterogeneities in both strain distribution and chemical composition.
The Science
Scientists have discovered a previously unseen nanoscale strain-relief mechanism in boehmite crystals, where hydrogen bonding drives the twisting of atomic layers.
The Impact
New insights into strain-driven transformation could help scientists tune the properties of materials used for flexible electronics, semiconductors, catalysts, and more.
Summary
When materials are squeezed, stretched, or heated, internal stresses develop and lead to deformation, known as “strain.” How strain is released at nanoscale dimensions isn’t well understood, but it strongly affects material performance in emerging technologies and in natural systems like rocks and minerals.
A research collaboration involving the U.S. Department of Energy’s Brookhaven National Laboratory, Lawrence Berkeley National Laboratory, Sandia National Laboratories, Stanford University, and the University of California, Berkeley, investigated this complex process. The team studied boehmite, a layered aluminum oxyhydroxide mineral that forms when another mineral, gibbsite, is heated and loses water. Unlike graphene and similar two-dimensional materials whose layers are weakly held together, boehmite’s layers are connected by hydrogen bonds, which are both flexible and directional.
Using advanced characterization tools, including in situ transmission electron microscopy at the Center for Functional Nanomaterials (CFN), X-ray diffraction and spectroscopy at three National Synchrotron Light Source II (NSLS-II) beamlines (Quick X-ray Absorption and Scattering, Pair Distribution Function, and X-ray Powder Diffraction), and machine-learning-based computer simulations at CFN and BNL’s Computing and Data Sciences directorate (CDS), the team was able to get a better understanding of this process. During the experiment, the researchers watched boehmite crystals in real time as they were heated to about 575 K (about 300°C/575°F). This allowed them to see exactly how strain developed and relaxed inside individual crystals. They found that instead of cracking or forming several defects, the crystals relieved strain as entire atomic layers slowly twisted relative to one another.
This twisting creates visible moiré patterns, which magnify tiny distortions in the crystal. By tracking how these patterns moved and changed, the team could measure how much the layers twisted and how fast strain was being released. Computer simulations showed that hydrogen bonds between layers play a key role. As layers twist by angles of about 5–10 degrees, the hydrogen bonds stretch, rearrange, and interfere with one another.
This study reveals a previously unknown strain-relief mechanism. These findings suggest that other hydrogen-bonded layered materials may also release stress by twisting their layers. This insight could help scientists design novel materials whose properties can be tuned by strain, gain a better understanding of mineral transformations, and improve materials for electronics, energy technologies, and catalysis.
Download the research summary slide (PDF)
Related Links
DOI: https://www.science.org/doi/10.1126/sciadv.ady6869
Contact
Haimei Zheng
Lawrence Berkeley National Laboratory
hmzheng@lbl.gov
Chun Chang
Lawrence Berkeley National Laboratory
chunchang@lbl.gov
Deyu Lu
Brookhaven National Laboratory
dlu@bnl.gov
Publications
Qi Zheng, Boyang Li, Sizhan Liu, Chuntian Cao, Jessica M. Rimsza, Qiubo Zhang, Jianming Bai, Chun Chang, Jiawei Wang, Chengyao Liang, Haiyan Mao, Matthew R. Carbone, Deyu Lu, Tatiana Pyatina, and Haimei Zheng, Strain release through hydrogen bond–mediated layer twisting.Sci. Adv.11,eady6869(2025). DOI:10.1126/sciadv.ady6869
Funding
This work was supported by the U.S. Department of Energy, Office of Science supporting Energy EarthshotTM Initiative, as part of the “Center for Coupled Chemo-Mechanics of Cementitious Composites for EGS (C4M)”. Part of the work was carried out as user projects at the Molecular Foundry at Lawrence Berkeley National Laboratory, a user facility also funded by the US Department of Energy under contract no. DE-AC02-05-CH11231. This research also used 7-BM (QAS), 28-ID-1 (PDF), and 28-ID-2 (XPD) beamlines at the National Synchrotron Light Source II (NSLS-II), the Theory and Computational resources of the Center for Functional Nanomaterials (CFN), and computational resources of the Scientific Data and Computing Center, at Brookhaven National Laboratory. NSLS-II and CFN are US DOE Office of Science User Facilities operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. Research was performed in part at the Texas A&M University System Center for Infrastructure Renewal (CIR). This article has been authored by an employee of National Technology & Engineering Solutions of Sandia LLC, under contract no. DE-NA0003525 with the US Department of Energy (DOE).
2026-22834 | INT/EXT | Newsroom









